Bio-imaging and photodynamic therapeutic treatment of cancers
by Dr. Sunaina Singh
ssingh@lagcc.cuny.edu
ABOUT THE AUTHOR

Dr. Sunaina Singh
Dr. Sunaina Singh received her Ph.D. from Hunter College of the City University of New York in the laboratory of Prof. Charles Michael Drain. She then pursued her postdoctoral research work with Dr. Ronald Koder at The City College of New York, CUNY. In August 2013, she joined as a faculty member at the department of Natural Sciences, LaGuardia Community College, where her research focuses on the synthesis of porphyrin-based photosensitizers for photodynamic therapy and imaging.
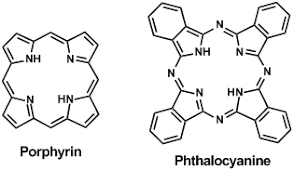
Figure 1: Structures of porphyrin and phthalocyanine macrocycle.
My research focuses on the development of new generation photosensitizers for bio-imaging and Photodynamic therapeutic (PDT) treatment of cancers. As deaths from preventable diseases abate, cancer is becoming one of the leading causes of death in the world. Photodynamic therapy (PDT) is a non-invasive treatment for cancer involving the interactions of light, a photosensitizer (PS), and oxygen that results in generation of highly reactive oxygen species (ROS). These ROS react with diffusion limited kinetics with a range of biochemical structures in cells to induce oxidative damage to the cell, thereby causing cell death via apoptosis or necrosis. PDT has been used as a treatment for a variety of cancers including bladder, brain, breast, skin, lung, esophagus, and bronchial cancer. Porphyrins and phthalocyanines are the most common and efficient photosensitizers (PSs) used in PDT because of their absorption in the visible region of the optical spectrum, long lived triplet excited state, and efficient phototoxicity towards cancer cells [1,2].
A number of requirements must be fulfilled for the development of new porphyrin photosensitizers, including chemical stability, the ease of administration, localization in tumor, the capacity to absorb at long wavelength in the red region of the optical spectrum to allow greater penetration of light into tissues, and minimal side effects. The uptake and localization of the photodynamic agent in the cell depends mainly on the exact chemical structure of the dye and any covalently bonded auxiliary groups [3,4]. The most commonly used and FDA approved porphyrin photosensitizer is Photofrin®, a derivative of haematoporphyrin (first generation drug). Meta-tetrahydroxyphenyl chlorin (m-THPC) is a second generation photosensitizer approved in Europe and some other countries. Photofrin® absorbs only weakly at about 620 nm, which does not match well with the optimal light wavelength (650-750 nm) for tissue penetration, and therefore is unsuitable for the deep seated tumors [5].
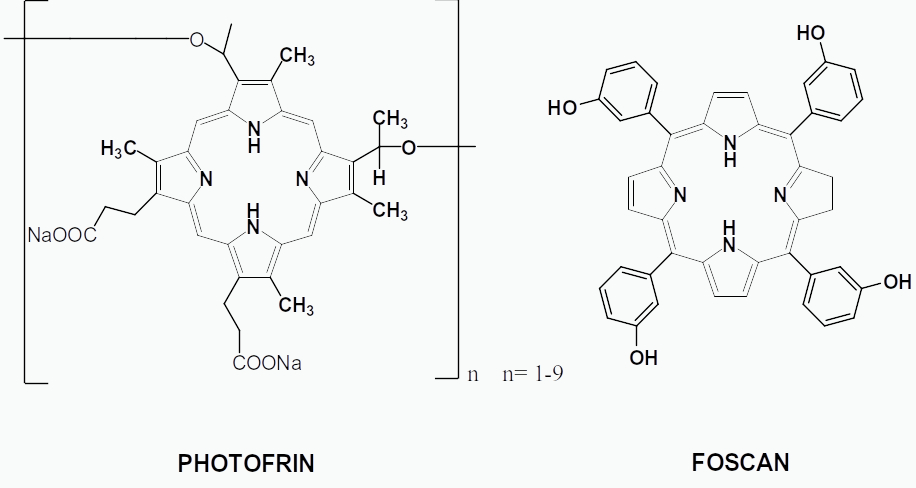
The m-THPC, trade name Foscan and Temoporfin, on the other hand is a second generation photosensitizer with several advantages over Photofrin®. The m-THPC is approximately 200 times more effective than Photofrin® when considering photodynamic dose (i.e. lower dose and shorter illumination time). Though m-THPC has several advantages over Photofrin®, the skin photo sensitivity caused by m-THPC is only slightly less than that of Photofrin®. Also, neither compound specifically targets cancer cells or tissues. Other criteria include that a good PDT agent should be amphiphilic so that it can easily pass through the cell membrane and is easily removed after the treatment. By attaching different polar groups on these dyes such as amino acids, sugars, peptides and polyethylene glycols, the polarity and the partition coefficient of these dyes can be altered [6,7].
In recent years, new methods have been developed to convert porphyrins into dihydroporphyrins (chlorins) and tetrahydoporphyrins (bacteriochlorins and isobacteriochlorins). These compounds have been found to exhibit stronger absorptions band in the red region of the optical spectra [8, 9].
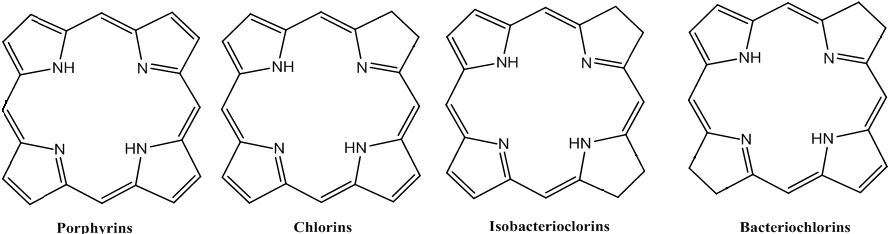
Similarly, phtahlocyanines can also be more efficient in generating reactive oxygen species than porphyrins. For therapeutic applications, phthalocyanines have strong electronic bands at the red region that are about two orders of magnitude stronger than that of porphyrins, thus enabling them to act deeper under the skin. Phthalocyanines are also extraordinary stable [10].
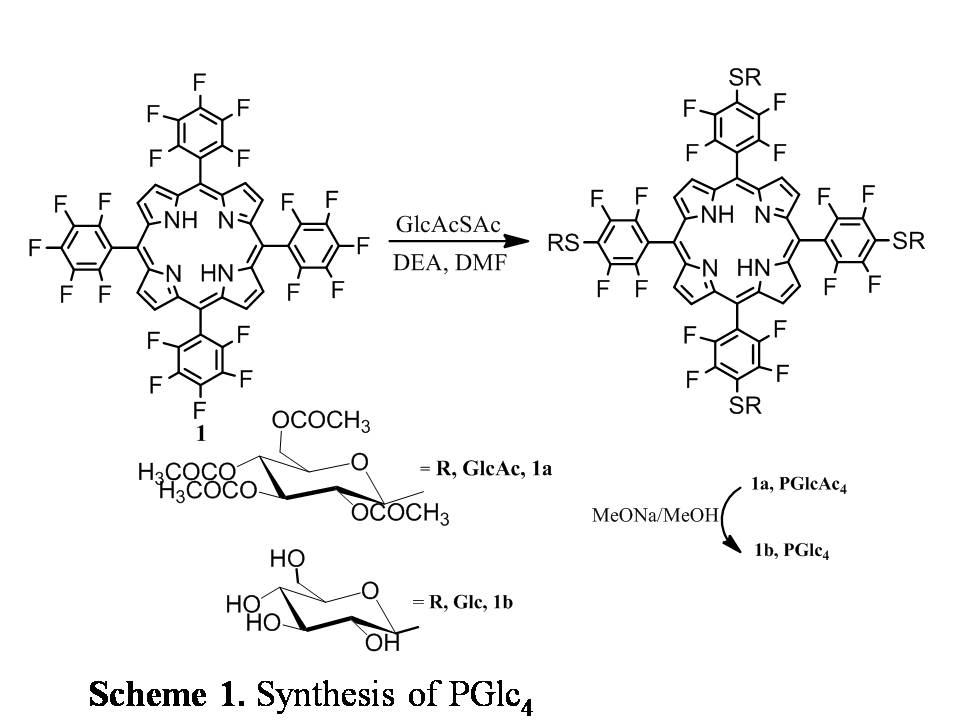
This work builds upon my thesis research where we made nonhydrolysable glycosylated porphyrins on a core porphyrin platform (Scheme 1). This design takes advantage of the fact that glycolysis is increased in the cancer cells as compared to the normal cells and thus increased glucose uptake. The sugar moieties on the porphyrin may mediate specific interactions with cancer cell membranes and active uptake of the compound [2]. The glucose porphyrin is quite selective and effective PDT agent in vitro using MDA-MB-231 human breast cancer and other cancer cell lines. The next step was to synthesize the glucose derivatives of the chlorin (CGlc4), isobacteriochlorin (IGlc4) and bacteriochlorin (BGlc4) systems [11]. The excited state lifetimes, singlet and triplet quantum yields, and distortion upon metal ion binding are significantly different for these three types of porphyrinoids.
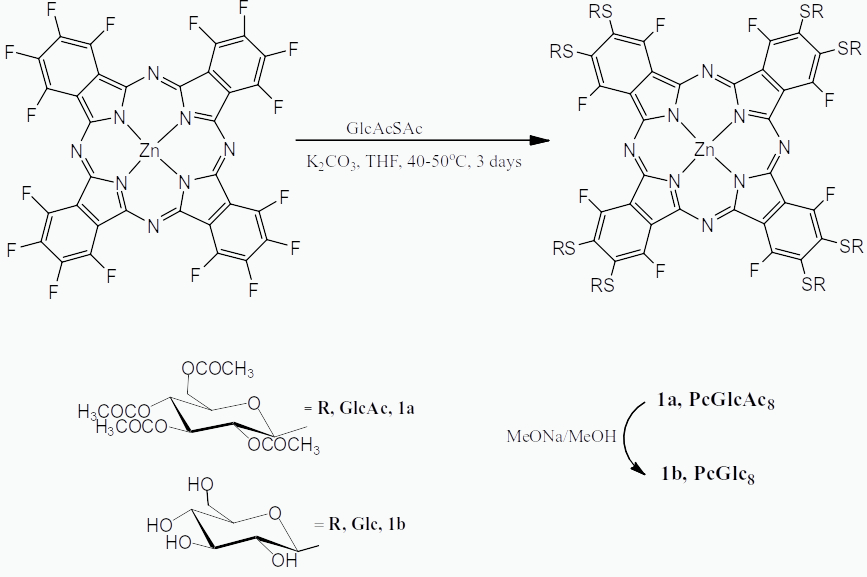
Scheme 2: Synthesis of Zinc Phthalocyanine substituted with eight thioglucose units [11].
Our group reported the formation of Zinc(II)phthalocyanine appended with eight thioglucose units via a nucleophilic substitution reaction where a base is used to substitute the peripheral fluoro groups on commercially available hexadecafluorophthalocyaninatozinc(II) (ZnPcF16) with thioglucose units (Scheme 2). This compound was found to be amphipathic, stable and has potential to be used as a photosensitizer in PDT [10].
Recently our group reported a comparison between a thioglycosylated chlorin and phthalocyanine as potential theranostic agents and we found that chlorin appended with thioglucose unit is more promising theranostic agent [12].
Currently, we are working on developing porphyrin based theranostic agents for dual functionality as photosensitizer for photodynamic therapy (PDT) and as contrast agent for magnetic resonance imaging (MRI).
References
1. Vazquez, M.S.; Jensen T.J.; Vicente M.G.H. J. Med. Chem. 2008, 51 (10), 2915-2923.
2. Chen, X.; Hui, L.; Foster, D. A.; Drain, C. M. Biochemistry 2004, 43, 10918-10929.
3. Pasetto, P.; Chen, X.; Drain, C. M.; Franck, R. W. Chem. Commun. 2001, 81-82.
4. Samaroo, D.; Vinodu, M.; Chen, X.; Drain, C. M. J. Comb. Chem. 2007, 9, 998-1011.
5. Hilmey, D.J.; Abe,M.; Nelen, M.I.; Stilts, C.E.; Baker, G.A.; Baker, S.N.; Bright, F.V.; Davies, S.R.; Gollnick, S.O.; Oseroff, A.R.; Gibson, S.L.; Hilf, R.; Detty, M.R. J. Med. Chem. 2002, 45 (2), 449-461.
6. Sternberg, E. D.; Bruckner, C.; Dolphin, D. Tetrahedron 1998, 54, 4151-4202.
7. Bonnett, R. Chem. Soc. Rev. 1995, 24, 19-32.
8. Silva, A. M. G.; Tome, A. C.; Neves, G. P. M. S.; Silva, A. M. S.; Cavaleiro, J. A. S. J. Org.Chem. 2005, 2306-2314.
9. Silva, A. M. G.; Tome, A. C.; Neves, G. P. M. S.; Silva, A. M. S.; Cavaleiro, J. A. S. Chem. Commun. 1999, 1767-1768.
10. Aggarwal, A.; Singh, S.; Zhang, Y.; Anthes, M.; Samaroo, D.; Gao, R.; and Drain. C. M. Tetrahedron Lett., 2011, 52, 5456-5459.
11. Singh,S.; Aggarwal, A.; Thompson, S.; Tome, J. P. C.; Zhu,X.; Samaroo, D.; Vinodu, M.; Gao, R.; Drain, C. M. Bioconjugate Chem. 2010, 21 (11), 2136-2146.
12. Singh, S.; Aggarwal, A.; Bhupathiraju, N. V. S. D. K.; Jovanovic, I.R.; Landress, M.; Tuz, M.P.; Gao, R.; Drain C. M. Bioorg. Med. Chem., 2020, 28, 115259.