by Gisela Ismaili and Dr. Lucia Fuentes
ABOUT THE AUTHORS

Gisela Ismaili
Gisela Ismaili graduated from LaGuardia Community College in Spring 2021, with a High Honors Degree and transferred to Hunter College to complete her studies in Biological Sciences. In her second year at LaGuardia, from Sept 2020-June 2021, she conducted research with Dr. Lucia Fuentes. Her research project received recognition at the 2021 CUNY Research Scholars Program Symposium where she received the Best Poster Presentation Award.

Dr. Lucia Fuentes
Lucia Fuentes is a Professor of Biology in the Natural Sciences Department at LaGuardia Community College. For the past twenty years, Dr Fuentes has taught and done research in Biology, particularly the areas of cell biology and immunology. She also teaches Anatomy and Physiology and General Biology for first year students.
Generally, brains of individuals suffering from traumatic injuries or degenerative diseases such as Alzheimer’s disease, have a compromised state of the Blood-Brain-Barrier (Vijaya Kumar et al., 2015).
The Blood Brain Barrier (BBB) is a selectively permeable membrane that prevents pathogens from circulating into the brain but also, at the same time, allows vital nutrients to enter the brain. Its principal units are the endothelial cells. A compromised state of the BBB allows pathogens such as viruses and bacteria as well as Yeasts like Candida, to reach the brain (Vijaya Kumar et al., 2015). The experimental set up we present here is based on the principles underlying the cellular composition of the BBB. We propose a compelling in-vitro model, which can be induced to mimic a compromised state of the BBB, allowing to study and analyze the reaction of microglia to such abnormal conditions. Microglia are the professional phagocytes of the Central Nervous System; they engulf wastes and foreign particles found in the brain.
In our model, we will use mouse-derived immortalized microglial cells, BV2 (Figure 1), while an Immortalized Human Brain Endothelial Cell Line, HCMEC/D3 (Figure 2) will be used to mimic the BBB cells.
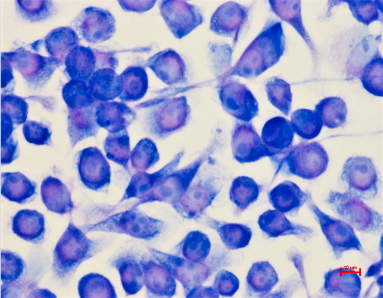
Figure 1. BV-2 Microglial Cells. Photo by Dr. Fuentes. |
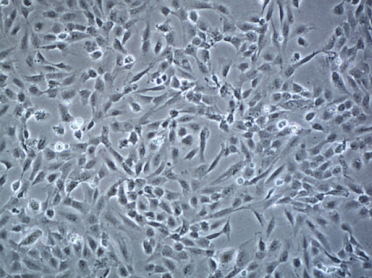
Figure 2. Immortalized Human Brain Endothelial Cell Line HCMEC/D3 (in-vitro Blood-Brain-Barrier). Photo by Sigma Aldrich. |
The novel setup, which is a modification of Vu, K., Weksler, B., Romero, I., Couraud, P. O., & Gelli, A. (2009) model, consists of a trans-well apparatus in which endothelial cells will be grown on a collagen coated porous membrane, in a rich endothelial growth medium; at the bottom of the well, beneath the endothelial layer, microglial cells will be seeded to adhere to the surface (Figure 3).
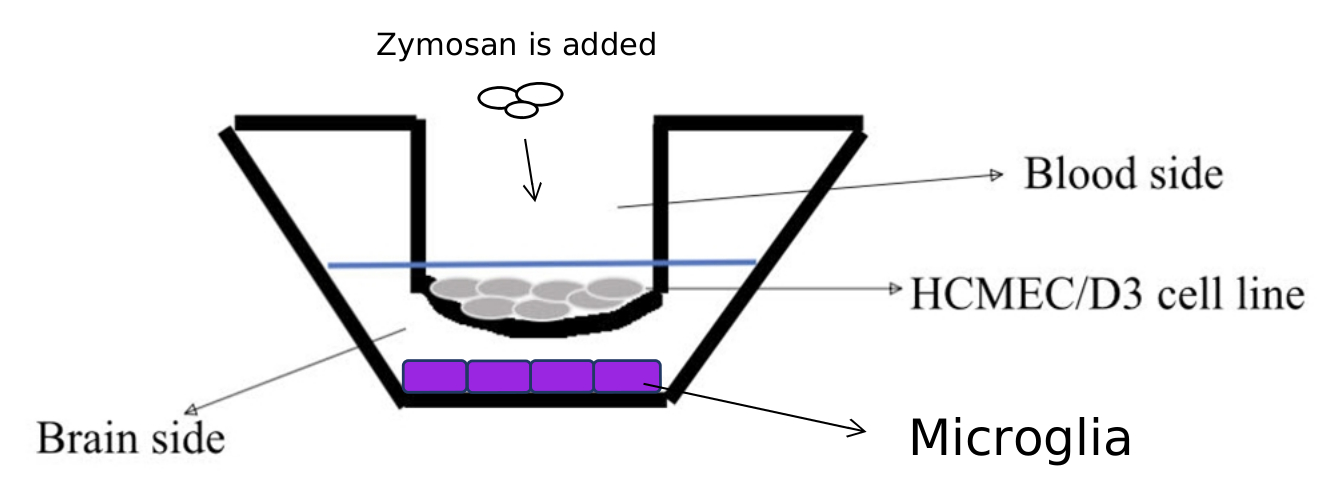
Figure 3. The Trans Well apparatus. |
The HCMEC/D3 cell line serves as the model of the Blood-Brain-Barrier, separating the “Blood” side from the “Brain” side, where microglial cells are.
To determine the utility of our setup for the study on the effects of disruption of the BBB on microglial activity, we propose experiments comprised of three stages. In the first stage, the HCMEC/D3 will be pretreated with Lipopolysaccharide (LPS), a bacterial component which promotes inflammation, before seeding the microglial cell on the bottom well. We will run controls by exposing the cells to all the components but without the LPS. In the second stage, we will be using zymosan, a yeast-derived particle which will mimic the effects of live yeast. After its addition, the changes of permeability of the BBB to zymosan will be determined. Zymosan along with LPS will mimic a compromised state of the BBB. In the final stage, after zymosan and LPS have been added to the endothelial cell line, the changes in microglia’s activity will be analyzed. Specifically, in the experiment, the phagocytic activity, release of pro-inflammatory molecules such as nitric oxide (NO), Tumor necrosis factor alpha (TNF-α), Interleukin 1 beta (IL-β) and IFN-γ (interferon gamma) will be measured. Moreover, the changes in the cellular anatomy of microglia will be observed.
In this article, we presented a system that provides an in-vitro alternative for the examination of the effects of changes in the BBB. It is expected that the insights gained by the results derived with this novel set up will contribute to a deeper understanding of the role of the BBB in the pathogenesis of AD, particularly on the changes in microglial function associated with this neurodegenerative disease.
References:
Vu, K., Weksler, B., Romero, I., Couraud, P. O., & Gelli, A. (2009). Immortalized human brain endothelial cell line HCMEC/D3 as a model of the blood-brain barrier facilitates in vitro studies of central nervous system infection by Cryptococcus neoformans. Eukaryotic Cell, 8(11), 1803–1807. https://doi.org/10.1128/EC.00240-09
Vijaya Kumar, D. K., Eimer, W. A., & Ramakrishnan, S. (2015). Specificity of Toll-Like Receptor 2 and Dectin-1 Signaling in CNS Macrophages. The Journal of neuroscience: the official journal of the Society for Neuroscience, 35(49), 16015–16017. https://doi.org/10.1523/JNEUROSCI.3453-15.2015