by Mengyi Lin (Guttman CC, Liberal Arts and Sciences, 2021-2022 CRSP cohort)
The work was done as a part of the CRSP program at Guttman Community College/CUNY, under the supervision of Dr. Chulsung Kim.
This article has been published as part of the Special Edition of Ad Astra, which features the CUNY Research Scholars Program (CRSP) across The City University of New York. The issue is accessible at http://adastraletter.com/2024/crsp-special-edition/.
ABOUT THE AUTHOR

Mengyi Lin
Mengyi Lin, a graduate of Guttman Community College who is now pursuing an architecture major at New York City College of Technology, conducted research under the guidance of Professor Chulsung Kim during the academic years of 2021 to 2022. This research endeavor, supported by the CUNY Research Scholars Program, revisited the DPC methods for measuring hexavalent chromium with a focus on statistical and kinetic perspectives. Mengyi presented the findings of this research at the American Chemical Society Middle Atlantic Regional Meeting.
Hexavalent chromium [Cr(VI)] is a potential carcinogenic pollutant found commonly in many industrial areas. Various assays have been developed and applied to determine the amount of hexavalent chromium in aqueous solutions. One of the standard methods adopted for the Cr (VI) quantitative analysis is the 1,5-diphenylcarbazide colorimetric (DPC) method following the EPA method 7196A guidelines. The process includes color development based on the redox reaction between hexavalent chromium and the diphenyl carbazide. A study has been performed to revisit the DPC method using statistical and kinetic perspectives. The experimental results indicated a significant dependency of the method's accuracy on the redox reaction duration. Notably, the coefficient of determination (R²) decreased as the reaction period was extended.
Chromium (Cr) is an essential mineral nutrient involved in metabolism. It predominantly exists in two forms: trivalent chromium [Cr(III)] and hexavalent chromium [Cr(VI)], with the form depending on environmental conditions. Cr(VI) is commonly produced in industrial processes and is used in dyes, paints, and pigments.
Water is essential for life, playing a critical role in the human body. It is required daily to maintain the proper functioning of all body parts, organs, and cells. However, the presence of toxic substances like hexavalent chromium [Cr(VI)] in water poses significant health risks, including carcinogenic potential, to living organisms, including humans. Due to the simultaneous presence of Cr(VI) and Cr(III) in typical environmental settings, the US Environmental Protection Agency (EPA) has established drinking water guidelines based on the total chromium content [1].
A standard method for quantifying aqueous Cr(VI) recommended by the EPA is the 1,5-diphenyl carbazide (DPC) assay [2]. In this assay, the introduced DPC complex with the aqueous phase Cr(VI) produces a purple-red color. The developed color was measured using colorimetry. The current EPA procedure recommends a 5-10 minute color developing period, and the absorbance of the fully developed color can be determined photometrically. The concentration of Cr(VI) is then calculated using a standard curve regression line equation derived from the least squares method with several different Cr(VI) concentrations. However, in many preliminary experiments, the DPC assay's redox reactions were inconsistent and fluctuated. Therefore, this study aimed to re-examine the DPC method to evaluate its accuracy and reliability.
Hexavalent chromium [Cr(VI)] concentrations were determined using the 1,5- Diphenylcarbazide (DPC) method, following the general procedure outlined in the EPA 7196A method [2]. Fresh 1% DPC reagent was prepared weekly by dissolving 1,5-diphenylcarbazide in acetone. A phosphate buffer solution was prepared by mixing 34.2 mL of phosphoric acid and 10 g of sodium hydroxide (NaOH) and then diluting it to 500 mL with deionized water. A 1000 mg/L Cr(VI) stock solution was prepared using potassium dichromate, from which standard solutions were prepared through serial dilution. All solutions were stored in a refrigerator. All chemicals used in the experiments were purchased from Fisher Scientific and were of ACS grade or higher.
To determine the optimal absorbance wavelength for Cr(VI) detection, a 15 mg/L Cr(VI) solution was prepared and scanned over a wavelength range from 380 nm to 800 nm using an Orion Aquamate UV-Visible spectrophotometer, following a 5-minute DPC-Cr(VI) color development period.
Various Cr(VI) concentrations, ranging from 1.5 mg/L to 15 mg/L, were prepared for the experiments. Different color development times, up to 16 minutes, were applied to identify the optimum conditions for aqueous phase Cr(VI) determination. The solutions were placed on a rotational shaker set to 30 rpm for uniform mixing during the color development process. All experiments were performed six times.
The EPA Method 7196A for the determination of aqueous phase Cr(VI) was re-evaluated in this study. Figure 1 presents the absorption spectrum, ranging from 380 to 800 nm, of the 15 mg/L Cr(VI)-DPC complex solution aged for 5 minutes. Contrary to the EPA's recommended wavelength of 540 nm, the peak absorbance in our study was observed at 522 nm. Consequently, all subsequent experiments were conducted at this wavelength of 522 nm.
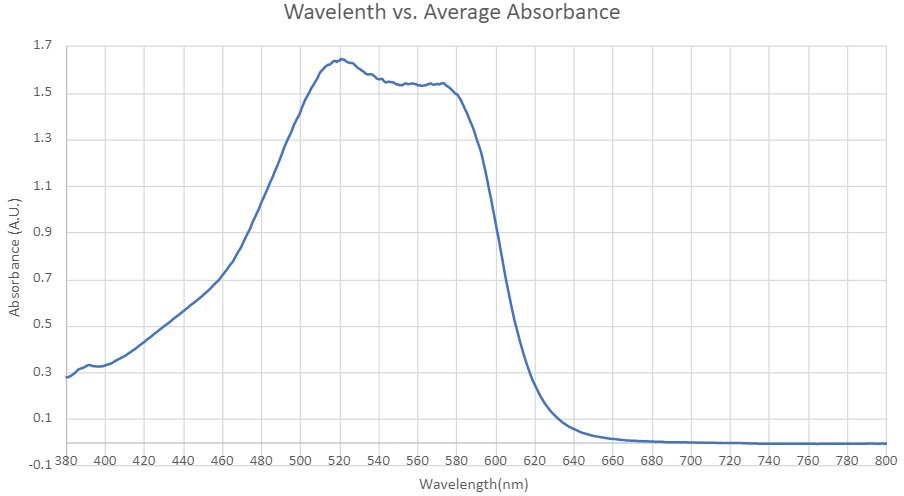
Figure 1: Spectrum of Cr(VI) solution in the range of 380 nm and 800 nm.
Figure 2 illustrates the change in absorbance as a function of color development time. This was tested across seven Cr(VI) concentrations, ranging from 1.25 mg/L to 15 mg/L. In all experiments, over 90% of the maximum color development occurred within the first 2 minutes. Beyond this, the absorbance values plateaued from 4 to 16 minutes, indicating no further significant color development. Additionally, the trend in absorbance as a function of time remained consistent across the different Cr(VI) concentrations.
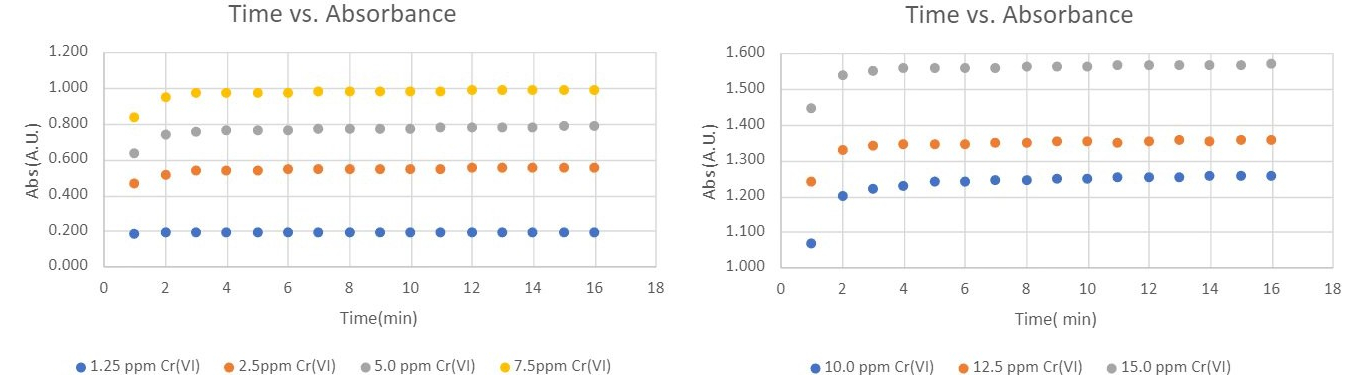
Figure 2: Absorbance changes as a function of color development time with different Cr(VI) concentrations.
While the absorbance values appeared to plateau after 4 minutes, a closer examination of the data revealed slight changes. Figure 3 (a) and (b) show the average absorbance value changes for 1.25 mg/L and 15 mg/L Cr(VI) solutions, respectively, after four minutes of color development. As displayed in Figure 3, for both concentrations, there was a continuous yet modest increase in absorbance throughout the 16-minute experimental period.
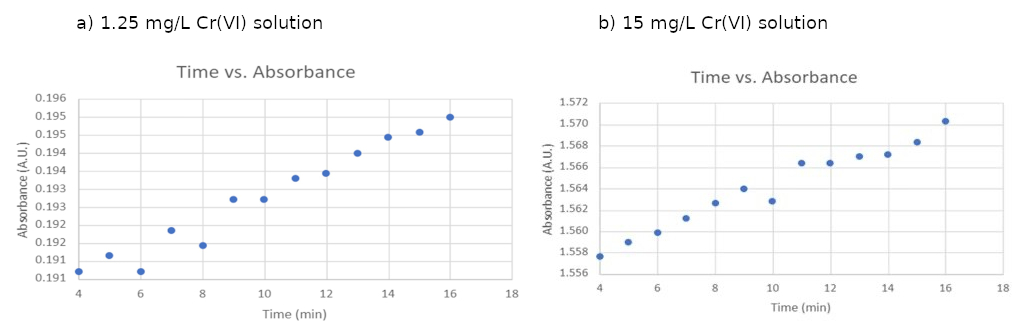
Figure 3: Zoomed in absorbances as a function of color development times in 1.25 mg/L and 1.5 mg/L Cr(VI) solutions.
Standard curves were constructed to related absorbance with Cr(VI) concentration over various color development times. The coefficient of determination (R2 values) was calculated by correlating known Cr(VI) concentrations with their respective absorbance measurements. R2 values, obtained from standard curve analyses at color development intervals ranging from 4 to 16 minutes, are presented in Figure 4. This figure demonstrates that, although there was no significant drop, the R2 values gradually decreased as the color development time increased.
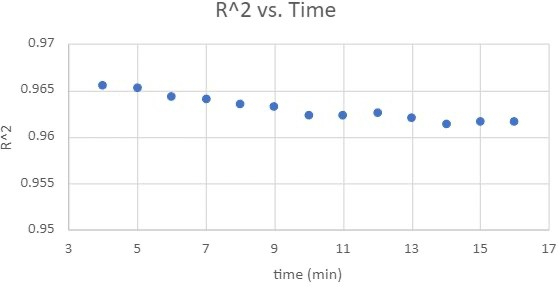
Figure 4: The coefficient of determination changes as a function of color-development time.
Given the unstable color development observed in the DPC assay of the EPA Method 7196A, a method over 30 years old, a thorough re-evaluation was undertaken. Experimental data indicated that a uniform color development reaction period is essential for all samples due to the continuous change in absorbance. It was observed that most color developments occurred within the first 2 minutes, consistently across all Cr(VI) concentrations. Although the changes were not significant, color development continued throughout the EPA's recommended 5 to 10-minute window. In addition, the accuracy of the standard curve diminished as the reaction time increased. Based on these findings, the optimal color development time for the DPC assay is suggested to be between 4 and 6 minutes, and this duration should be consistently maintained throughout the experiment.
[1] Environmental Protection Agency. (n.d.). EPA. Retrieved July 20, 2022, from https://www.epa.gov/sdwa/chromium-drinking-water#if-epa-decides-regulate
[2] Chromium, Hexavalent (Colorimetric). U.S. Environmental Protection Agency. Retrieved Feb, 2022, from https://www.epa.gov/sites/default/files/2015-12/documents/7196a.pdf