by Ellis Spica (Guttman CC, Liberal Arts and Sciences, 2022-2023 CRSP cohort)
The work was done as a part of the CRSP program at Guttman Community College/CUNY, under the supervision of Dr. Chulsung Kim.
This article has been published as part of the Special Edition of Ad Astra, which features the CUNY Research Scholars Program (CRSP) across The City University of New York. The issue is accessible at http://adastraletter.com/2024/crsp-special-edition/.
ABOUT THE AUTHOR

Ellis Spica
Ellis Spica is a graduate of the Liberal Arts and Sciences major at Guttman Community College and is currently studying in the Health Sciences department at Stony Brook University. He received his associate degree in June of 2023. During his sophomore year at Guttman, Ellis engaged in research under the mentorship of Dr. Chulsung Kim in the Science Program, studying a statistical evaluation of the presence of hexavalent chromium in drinking water, focusing on its detection in the presence of potassium chloride. He presented his research at the 2023 ACS Northeast Regional Meeting in Boston with the support of the CUNY Research Scholars Program (CRSP). After graduating with a bachelor's degree, Ellis plans to continue his education in the medical field.
Hexavalent chromium is a highly soluble and carcinogenic substance, and its presence in drinking water is a significant health concern. Accurate and timely detection of [Cr(VI)] in water is vital for water safety assurance. While there are various methods for detecting [Cr(VI)] in its aqueous phase, they often involve high costs and extended timeframes. Recent advancements have introduced a direct method for determining [Cr(VI)] in water by measuring absorbance at a wavelength of 325 nm. This study aimed to analyze the determination of hexavalent chromium in the presence of potassium chloride, a common ion. In the experiment, varying concentrations of potassium chloride [KCl] were added to a consistent hexavalent chromium concentration of 10 mg/L. Each test was replicated at least ten times. The Grubbs test was used to identify and remove outliers from the data sets prior to statistical analysis. Student's t-tests were conducted for mean comparisons to evaluate the influence of KCl on the direct detection of [Cr(VI)] in its aqueous phase. The findings revealed that at concentrations up to 0.1 M KCl, there was no significant statistical impact on the direct determination of [Cr(VI)].
Chromium is a naturally occurring transition metal found in various environments. It predominantly exists in two oxidation states: trivalent chromium [Cr(III)] and hexavalent chromium [Cr(VI)]. Cr(III) is the more stable form of chromium, naturally present in both organisms and inanimate objects [1]. It has low solubility and is an essential nutrient for normal human bodily functions. In contrast, Cr(VI) is typically produced through industrial processes. This form is highly soluble and recognized as a carcinogen, posing significant risks to both human health and the environment due to its strong oxidizing properties. The tendency of hexavalent chromium to leak into the environment, often due to inadequate storage or waste management practices, has been a concern highlighted by past incidents [2].
According to the CDC and WHO, the average concentration of chromium in most drinking water in the US is less than 0.05 mg/L. The WHO sets the maximum permissible level of total chromium in drinking water at 0.1 mg/L [3]. This standard varies between countries and encompasses the combined total of both Cr(III) and Cr(VI), due to their ability to interchange under different oxidation and reduction conditions. Given its high toxicity and widespread presence, monitoring chromium levels in water is crucial for public health. Statistically, people living near areas with high Cr(VI) contamination in water sources exhibit noticeably poorer health, with reported impacts on gastrointestinal, hematological, and other bodily functions [4]. Therefore, continuous monitoring of Cr(VI) levels in drinking water is essential.
The rapid increase in industrialization has significantly heightened water pollution. Many developing countries experience greater impacts compared to developed nations, often due to differences in environmental regulations. Consequently, the concentration of Cr(VI) and other carcinogens has risen in some of these areas. A notable incident occurred in 2013 in Edogawa, Tokyo, where an abnormally high concentration of chromium was detected in a park. The highest recorded level was over 740 times the Japanese regulatory limit for chromium (5 μg/L). This spike was attributed to historical illegal dumping by a chromate plant. Despite the plant's closure nearly fifty years earlier, rainfall and snowmelt exacerbated the chromium pollution [5].
Various methods like gas chromatography, ion chromatography, spectrophotometry, high-performance liquid chromatography (HPLC), and differential pulse polarography are employed to detect hexavalent chromium in aqueous environments [6]. However, each of these methods has its limitations. Gas chromatography is suitable primarily for lower chromium levels due to its detection limit of 0.1μg/L, and its effectiveness depends on the optimization of solvent extraction and other instrumental parameters [7]. Differential pulse polarography, useful for determining hexavalent chromium concentrations in both natural and wastewater, offers a broad detection range from 10μg/L to 5.0mg/L [8]. However, its accuracy hinges on the size of the mercury drop used in the process.
While these methods are effective, they can be both time-consuming and costly. Even the relatively faster method, ion chromatography, requires over fifteen minutes and additional chemicals to detect hexavalent chromium. The goal of this research is to develop a rapid, cost-effective method that can be implemented on-site for immediate water analysis.
The research aimed to investigate the impact of KCl on a direct, rapid, and cost-effective method for detecting hexavalent chromium in water samples. A standard curve was created to establish a baseline using various concentrations of Cr(VI) without KCl. This curve was then used to compare changes in Cr(VI) concentration in the presence of KCl. Solutions of KCl ranging from 0.1M to 0.5M were prepared and combined with the Cr(VI) solution. The experiments were repeated ten times at each KCl concentration to enhance statistical accuracy. Outliers were identified and removed using Grubbs' test, ensuring reliability in the statistical comparison. The experimental data, both with and without KCl solution, were analyzed using the mean value t-test to assess the influence of KCl on the direct detection process of hexavalent chromium. All chemicals utilized in the experiments were of ACS grade or higher. Preliminary experiments established that a wavelength of 325 nm is most sensitive for detecting hexavalent chromium solutions. Consequently, all subsequent experiments were conducted using an Orion AquaMate 8000 UV-Vis spectrophotometer set at 325 nm. A blank solution was employed to calibrate the spectrophotometer to the correct baseline absorbance.
The outliers in the collected data were identified for each data group using the Grubbs test, as outlined in Eq. 1 [9] \begin{equation}\tag{1} g = \frac{|\mbox{questionable value}-\bar{x}|}{s}, \end{equation} where and \(s\) represent the average and standard deviation, respectively.
When the computed g value exceeded the critical g value at a 95% confidence level, the data point was identified as an outlier and subsequently excluded from further calculations. In cases where the critical g value was not available for a specific sample size, the critical g values for the two closest sample sizes (one larger and one smaller) were used. From these, a slope was determined, which then allowed for the calculation of an appropriate g value for the specific sample size used in this study. The calculation of \(S_{Pooled}\) is necessary to calculate the individual t values of the data sets. When the t-test value is greater than 95% confidence level student’s t value, the compared groups are considered statistically different, rejecting the null hypothesis of no significant difference in mean values. \begin{equation}\tag{2} t = \frac{|x_1-x_2|}{S_{Pooled}}\sqrt{\frac{n_1n_2}{n_1+n_2}}, \end{equation} where \(S_{Pooled}\) is a pooled standard deviation \begin{equation}\tag{3} S_{Pooled} = \sqrt{\frac{S_1^2(n_1-1)+S_2^2(n_2-1)}{n_1+n_2-2}}, \end{equation} here \(S_1\) and \(S_2\) are the standard deviations of the control, and the tested trial, respectively. The \(n_1\) and \(n_2\) denote the number of trials in the control, and in the tested group, respectively.
The correlation between the absorbance at 325 nm and the concentrations of Cr(VI) without KCl is shown in Figure 1. The absorbance is directly related to the amount of Cr(VI) in aqueous solution and the obtained r2 value and the regression equation are 0.9994 and Abs = 0.0187[Cr(VI)] - 0.0174. The [Cr(VI)] indicates the concentration of Cr(VI) in mg/L.
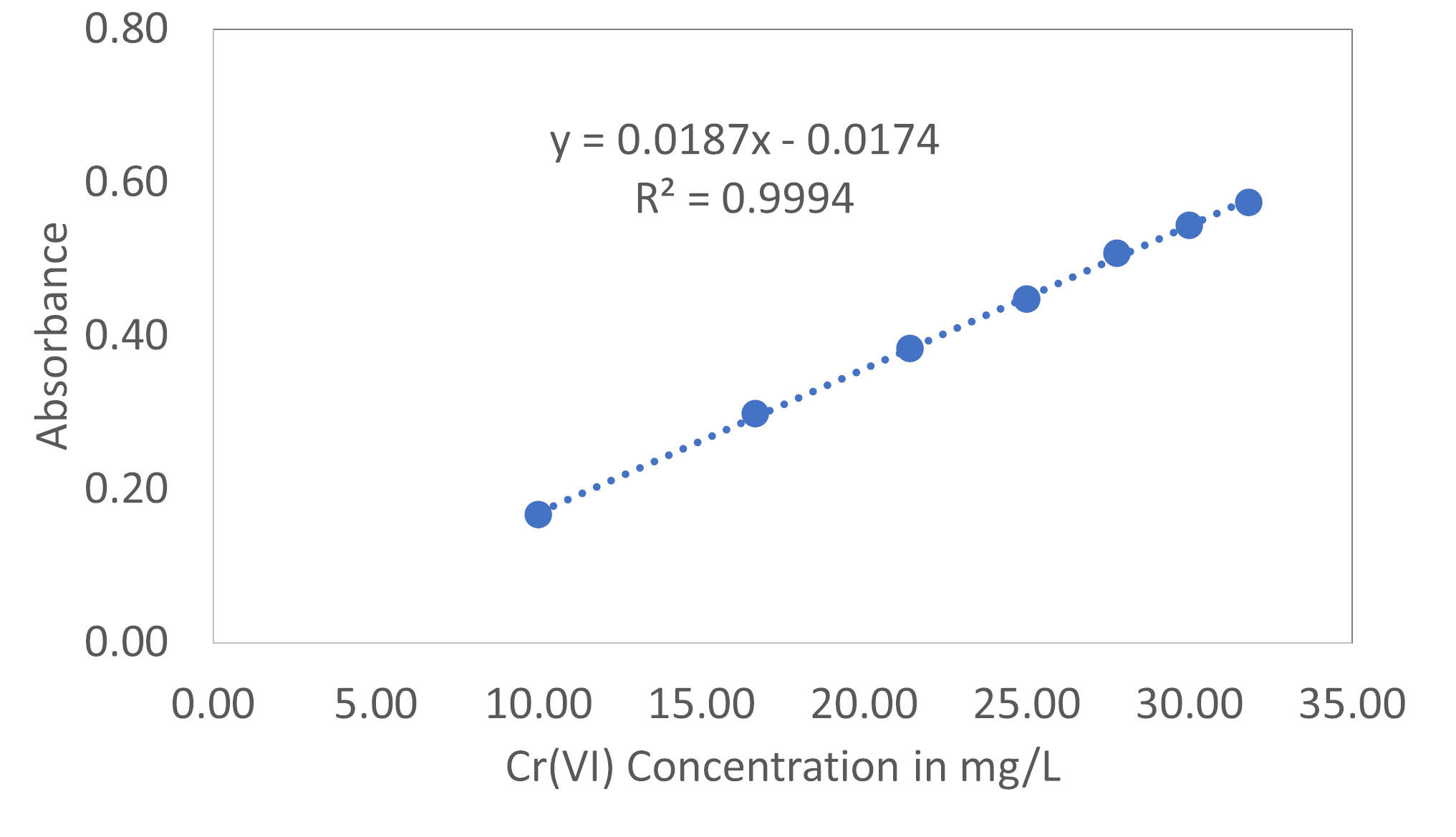
Figure 1: The correlation between Absorbance and the Cr(VI) concentration.
Each row of Table 1 represents the tested KCl concentration and whether it was accepted or not, alongside the number of outliers in the set. Any outliers were excluded from the data pool, and then the t-test was applied to each row to determine if the data was acceptable. Repeats in the [KCl] molarity were caused by the difference in the final concentration of the Cr(VI) solutions during calculations. As a result, certain molarity levels were tested more than once. The level of Cr(VI) present in the solutions did not have a significant impact on the results, however, an increase in the molarity of the KCl did produce a significant difference in the absorbance of the solutions.
| [Cr(VI)] mg/L | [KCl] M | Accepted | # of Outliers |
|---|---|---|---|
| 10 | 0.005 | NO Statistically Different between with and without KCl. |
0 |
| 10 | 0.010 | 1 | |
| 10 | 0.015 | 0 | |
| 10 | 0.020 | 0 | |
| 10 | 0.025 | 0 | |
| 10 | 0.030 | 1 | |
| 10 | 0.035 | 1 | |
| 10 | 0.040 | 0 | |
| 10 | 0.045 | 0 | |
| 10 | 0.050 | 0 | |
| 10 | 0.060 | 0 | |
| 10 | 0.070 | 0 | |
| 10 | 0.080 | 1 | |
| 10 | 0.090 | 0 | |
| 10 | 0.100 | 0 | |
| 10 | 0.125 | Statically Different between with and without KCl | 0 |
| 10 | 0.150 | 0 | |
| 10 | 0.175 | 1 | |
| 10 | 0.200 | 0 | |
| 10 | 0.225 | 0 | |
| 10 | 0.250 | 0 |
According to the experimental data, the direct detection method for the aqueous phase Cr(VI) is a rapid and convenient assay. There was no significant number of outliers identified, indicating that the direct method adopted for this research was a stable method. Comparison of the experimental t-values with the critical values from the student’s t-test at a 95% confidence level revealed: a) no statistical difference in Cr(VI) detection at KCl concentrations below 0.100 M; b) a statistical difference in Cr(VI) detection at KCl concentrations between 0.125 M and 0.2 M, compared to the absorbance without KCl. Further investigation is required to ascertain which ion, K+ or Cl-, is the primary interfering agent in the direct detection method at concentrations above 0.1 M.
[1] Chromium in Drinking Water. EPA, Environmental Protection Agency. https://www.epa.gov/sdwa/chromium-drinking-water.
[2] Chromium Release in Huron River Update. https://www.a2gov.org/departments/water-treatment/Pages/Chromium-Spill-.aspx.
[3] US EPA, O. W. National Primary Drinking Water Regulations. 2015. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations.
[4] Sharma P, Bihari V, Agarwal SK, Verma V, Kesavachandran CN, Pangtey BS, Mathur N, Singh KP, Srivastava M, & Goel SK. (2012), Groundwater contaminated with hexavalent chromium [Cr (VI)]: a health survey and clinical examination of community inhabitants (Kanpur, India). PLoS One, 7(10):e47877. https://doi.org/10.1371/journal.pone.0047877.
[5] Hori, M., Shozugawa, K. & Matsuo, M. (2015), Hexavalent chromium pollution caused by dumped chromium slag at the urban park in Tokyo. J Mater Cycles Waste Manag 17, 201–205. https://doi.org/10.1007/s10163-014-0243-0.
[6] Spinazze, A., et al. (2022). On the Determination of Cr(VI) in Cr(III)-Rich Particulates: From the Failure of Official Methods to the Development of an Alternative Protocol. Int. J. Environ. Res. Public Health, 19(19), 12111; https://doi.org/10.3390/ijerph191912111.
[7] SW-846 Test Method 7198: Chromium, Hexavalent (Differential Pulse Polarography). EPA, Environmental Protection Agency, 30 Jan. 2017. https://www.epa.gov/sites/default/files/2015-12/documents/7198.pdf.
[8] Lovett, R., & Lee, G. (1976). Analysis of chromium in natural waters by gas chromatography. Environmental Science & Technology. 10(1). 67 – 71. https://doi.org/10.1021/es60112a004.
[9] Stephanie Glen. “Grubbs’ Test for Outliers (Maximum Normed Residual Test)” From Statisticshowto.com: Elementary Statistics for the rest of us! https://www.statisticshowto.com/grubbs-test/.