by Dr. Sarah E. Durand (sdurand@lagcc.cuny.edu)
If you visit the LaGuardia College “C” (central) building, walk to the back of the building at 47th Avenue. Can you see the waterbody on the other side of the street? Upper floors give a better view. The water you see flows into the small river crossed by the Pulaski, Greenpoint Avenue and Kosciusko bridges. The river flows for four miles through the great City of New York. It’s named Newtown Creek and brings fish, crab, ducks and geese to live beside the people of west Queens and north Brooklyn. Newtown Creek is now a walled-in waterway through the city. But there were vast tidal meadows of the marsh grass Spartina alterniflora that once extended from the shores of the little river, covering what are now neighborhoods of Long Island City, Hunters Point, Maspeth and Greenpoint (Fig. 1). This tidal marsh was an ecosystem as biologically productive as a rain forest. [1]

Figure 1: Reconstruction of pre-colonial geography by artists, scientists, historians, and technicians of the Wilekia Project. The insert shows an aerial view positioned directly above Newtown Creek, wherein the dotted outline indicates the location of LaGuardia college and star indicates the location of the former shallow flat named Mussel Island. [2]
The marsh was regularly flooded by the tides, waves driven across the oceans by the gravitational forces of moon and sun. Twice daily a tidal wave passes through New York Harbor and Long Island Sound, rising and falling over roughly twelve hours. The wave travels up the rivers of the New York-New Jersey estuary, up the great Hudson River, up the smaller Passaic and Bronx Rivers and up the East “River,” from where the tidal wave enters the little waterway between Brooklyn and Queens (Fig. 2).

Figure 2: At low tide, the mouth of Newtown Creek, where it meets the East River, perhaps looked like this. Photo credit: Shutterstock
The crest of the wave, “high tide,” left only the grass tops of the marsh visible above the water. As the wave fell back over the following six hours, it exposed mud and sand flats beneath the grasses - places full of life. Fiddler crabs emerged from their burrows, the males signaling their territory location by waving one oversize claw (Fig. 3A). Softshell clams pulled back their extended siphons, leaving the paired inhalant and exhalent openings like two eyes in the mud. Thousands of shore birds landed on the flats as the tidal wave receded. They pulled up predatory worms (relatives of earthworms) and ran down small crustaceans (like the fiddler crabs). Racoons and possum came from the surrounding woodlands to forage on the clams (which they dug up) and on the ribbed mussels, Geukensia demissa, that were everywhere. But they were much more difficult to pull from the flats – they had used their foot to lay down exceptionally strong threads of protein (byssal threads) to anchor themselves to neighbors and to any nearby grass stems (Fig. 3B).
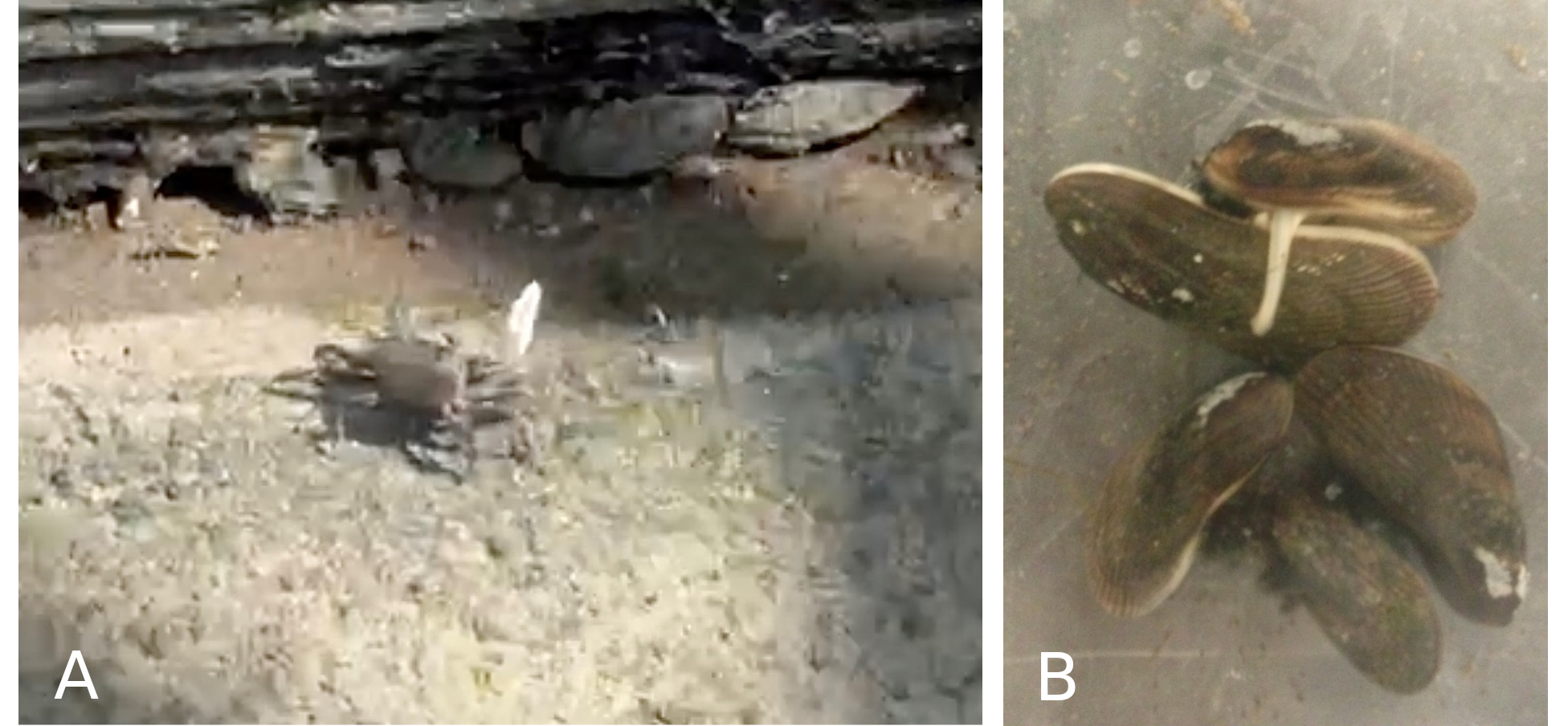
Figure 3: A: Fiddler crab, North Henry Inlet, Newtown Creek and B: Ribbed mussels in an aquarium: at right, a mussel extends its foot across the shell of a neighbor as it lays down byssal thread attachments. Photos by author.
The network of mussel threads and grass roots stabilized the sediments of the flats. The mussel shells provided hard attachment surfaces for “sessile” (non-mobile) animals, such as barnacles and anemones, and for seaweeds too (Figs. 4, 8). Barnacles are small crustaceans (like the fiddler crabs), but barnacle larvae leave the world of mobility as they settle out of the water and onto an available hard surface – like mussel shell. The young barnacles proceed to cement their heads onto the hard surface and undergo metamorphosis into a sessile adult that builds a conical white plated shell around its body. The shell has two plates at the top that close tightly as the water drains from the flats. Anemones can be thought of as upside-down jelly fish attached to hard substrate, again offered by mussel shell. As the tide withdraws, anemones pull water into their gut, retract their tentacles, contract their bodies into a soft bump and secrete a protective mucous.

Figure 4: Ribbed mussels in an aquarium two days following collection from the estuary: A: Circle indicates attached barnacle; B: Circle indicates attached anemone. Photos by author.
At some locations in the Newtown Creek estuary of 1600, generations of mussels had formed “mussel mounds”: one generation of mussel larvae after another settled on the shells of an earlier generation. Look at the aerial view of the reconstructed 1600 Creek (Fig. 1). Do you see Mussel Island? The flats here hosted the largest mussel mounds of the waterway. As mussel mounds were submerged by a rising tide, the water-filled spaces between the shells of adjacent animals were visited by juvenile fish, crab and shrimp taking refuge from predators. These small swimmers also found a bite to eat, thanks to a host of micro-organisms living on shell surfaces. The cavities of the mound and the thickets of grass stems were ideal hiding spaces for small aquatic animals to forage and grow. Indeed, the tidal marsh was a nursery for many species. As the tidal wave receded, the small swimmers followed the water into the safe crevices of subtidal oyster shoals.
Waves of human settlement coming from Europe began the great transformation. Over a century and a half, sediment dredged from the bottom of the Creek and its tributaries became fill to bury the marsh. The waterways were deepened – “channelized” – to allow transit of large commercial vessels; the living “soft” shore of grass and sediment was obliterated with a “hard” shore of seawalls called bulkheads. The walls made easy the delivery of materials and export of products. A legion of industrial establishments came to line the waterway and block its shores from the surrounding community. The Creek became a waste receptacle and transport service. Waste was not only industrial. Sewer lines were laid down underground at the speed that little creeks feeding the waterway were buried. The sewer lines connected with street drains. Pipes carrying combined street and sewer waste emptied into the waterway. An estuarine ecosystem, the tidal wetland, dwindled and then disappeared (Fig. 5).

Figure 5: Low tide at mouth of Whale Creek, Newtown Creek tributary in Greenpoint, 1939. Marsh grass remnants visible in foreground. Degnon Terminal, in background, was served by barge-to-rail from Newtown Creek’s Dutch Kills tributary in Long Island City. Photo credit: NY Public Library Digital Collection.
Industrial waste was eventually dominated by petrochemicals discharged or dumped or spilled by oil and gas industries. Standard Oil – to become Exxon, then ExxonMobil – came to Newtown Creek in the late 19th century as Standard Oil and established the Creek as a major center of oil refining and distribution on the East Coast. The notorious Greenpoint Oil Spill, identified as the largest land-based “spill” in US history, was actually a black lake of millions of gallons of oil beneath the streets. The oil is now being sucked back out by an ExxonMobil remediation project, initiated in large part by community pressure on the NYS Department of Environmental Conservation. [3]
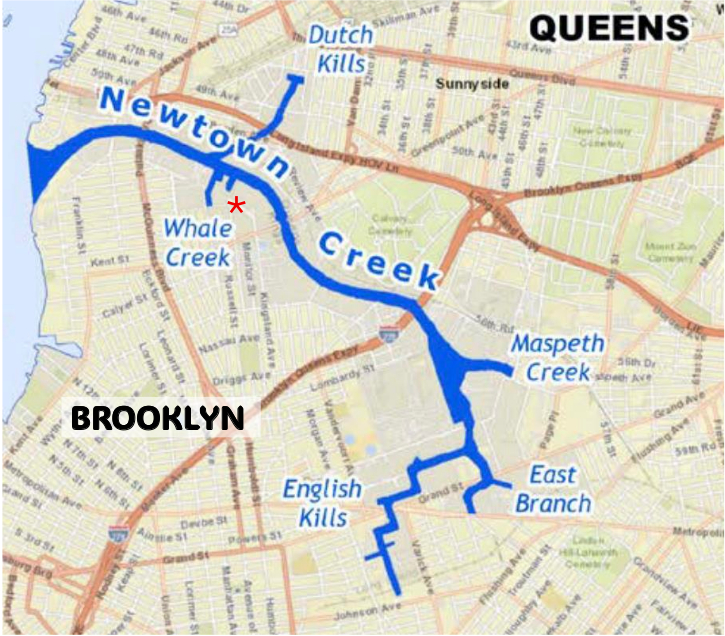
Figure 6: Newtown Creek Tributaries: Dutch Kills, Whale Creek, Maspeth Creek, East Branch, English Kills. Star indicates North Henry Street inlet. Courtesy of Newtown Creek Natural Resource Damage Assessment Trustee Council.
The underground black “lake” had resulted from decades of oil transport, refining, storage and delivery operations that leaked and spilled the product. It was discovered in 1978 by a Coast Guard helicopter as oil seeped from the shore and into the waterway as a black plume. Other reservoirs of petrochemical waste were subsequently identified along the shore and in the sediments. [3]
There also developed a high-volume inflow of biological waste to the waterway from growing residential communities. Now sewage enters the waterway as “combined sewage overflow” (CSO) via pipes that receive flow from both sewers and street runoff. CSO discharges contain dangerous substances, including pathogenic bacteria, pharmaceutical products and pesticides. Fecal matter in sewer inflow is fertilizer for the single-celled algae of the plankton - cells that photosynthesize like plant cells. With the fertilizer, the algal cell populations explode into “harmful algal blooms” (HABs). At night, these cells draw the oxygen from the water, leaving it hypoxic. And when the fertilizer from the CSO discharge is exhausted, the billions of floating algal cells die. They are decomposed by bacteria that draw even more oxygen from the water, leaving little or none for aquatic animals. At the head of each tributary (Fig. 6), excepting Whale Creek, is a major CSO outfall. Together, they discharge 90% of an estimated annual 1.2 billion gallons. The City’s Department of Environmental Protection (NYCDEP) is engaged in a massive infrastructure upgrade to reduce this volume.

Figure 7: Aerial photos at Maspeth Creek, arranged as time series, track transformation of Newtown Creek into a walled-in waterway. Underlying photo shows petroleum (photo by author). Aerial photos courtesy of Lionel Pincus and Princess Firyal Map Division, The New York Public Library.
The transformation of the Newtown Creek estuary was driven by a European culture of industrial capitalism. This new culture replaced the indigenous Lenape culture that had thrived in the estuary and its surrounds for thousands of years. Lenape industries of home building and food generation - rotational agriculture, sustainable shellfish harvesting, forest management - rested upon principles of environmental stewardship; the living world was considered part of the human community. [4, 5] In contrast, the colonizers prioritized the discovery of ecosystem elements to exploit for capital – resource accumulation. Celebration of resource accumulation, known as “profit,” replaced celebration of successful resource distribution within the community. The new culture promoted the goal of effective environmental exploitation over effective environmental stewardship. Under this new culture, “nature” was seen as separate from human domains of industry and residence – and sometimes “nature” had to be sacrificed. The consequences of this new culture were noted above.
Several types of hardened shore (Fig. 7) now bound Newtown Creek: modern steel sheet-pile walls, concrete, old wood bulkhead from the late 19th and early 20th centuries and “rip-rap” – a shoreline of human-placed boulders and chunks of concrete. Remarkably, in spaces between boulders where sediment collected, and inside the crevices of old wood walls or crumbling concrete bulkhead, an animal of the lost intertidal wetland had survived. The animal was the ribbed mussel. It is considered by ecologists as one of two “foundation species” of our East Coast salt marsh community for the reasons reviewed in Part 1 above. The other foundation species of two identified for the North American coastal wetland community is, of course, the grass species of the tidal marsh, Spartina alterniflora. This grass is also known as cordgrass because the Lenape people used the dried blades to weave “cordage” – rope.
As late as 2011, a biota survey contracted by the NYC Department of Environmental Protection failed to report the existence of the ribbed mussel, Geukensia demissa. The reason? A simple one: the biota survey failed to include surveys of the intertidal zone, the vertical distance between high tide and low tides along the shore. Why was this area omitted? Because it was assumed that no intertidal life could exist here given its habitat, the marsh wetland ecosystem, had been destroyed.
Can we restore some of a lost ecosystem? Yes indeed! Newtown Creek is alive. Floating in its water are the young of invertebrate and vertebrate animals of a marsh community. Juvenile fish – the vertebrate animals - and juvenile invertebrates: shellfish (e.g. mussels, oysters), crustaceans (shrimp, crab), worms, anemones and many others. Many of these young animals may begin life in another part of the larger estuary beyond Newtown Creek. As each develops from its fertilized egg, it is carried by current and tide into the walled-in waterway. This floating community, called plankton, also carries spores of seaweeds: brown seaweed like bladderwort, green seaweed like sea lettuce (Ulva) and a red seaweed called Graciliaria. The seaweeds are macroalgae (multicellular). Other algae species of the plankton are single-celled organisms invisible to the naked eye – and they never leave the plankton community. They are food for both the juvenile animals floating with them and for any clam, mussel or oyster - the bivalve mollusks called shellfish. Bivalve animals draw in water over their gills with their inhalant siphons. The algal cells are trapped – or “filtered” - out of the water and into streams of mucous heading for the mouth. The filtered water is then expelled through the animal’s exhalant siphon (Fig. 8).

Figure 8: Ribbed mussels in an aquarium. Mussel at left shows smaller exhalant siphon (inferior) and above it the brown-fringed tissue surrounding the inhalant siphon. Photo by author.
Can we expand the area of settlement substrate for larval mussels (Geukensia demissa) and, thereby, the aquatic life that would follow them? What if we increased the existing mussel population by an order of magnitude, from hundreds of thousands to millions? Success would bring a cascade of positive feedback effects to heal the estuary ecosystem: living space created on and between shells for other intertidal organisms; a greater volume of water filtered of the single-celled algae that can cause HABs [6]; and even the bacterial cells released by CSO discharges, such as Escherichia coli and Enterococcus, could be effectively filtered from the water. [7]
We must consider how to use the bulkhead walls of our waterway to create living space for its aquatic community of the lost marsh. The hard and homogeneous vertical surfaces of a bulkhead can be colonized by only some sessile filter-feeding animals other than bivalves (albeit an occasional oyster is successful, but this bivalve is subtidal). The invertebrate colonists of wall surfaces are barnacles, tunicates and sponges, animals that cannot effectively create much living space for other invertebrates and are largely confined to the subtidal zone. They exist as a single living layer on the wall surface. The soft tunicate and sponge bodies cannot support the settlement of other species and a barnacle’s conical shell is unsuitable for settlement, given its limited surface area and slope. But recall that mussels are present where there’s the slightest opportunity to anchor themselves between two opposing surfaces - and wherever a horizontal surface can accumulate sediment (recall the sand and mud flats of the lost marsh).
What if we suspend a settlement substrate from the top of a bulkhead? Or perhaps we can attach habitat to a wall’s surface? And can we not float living space? Lastly, where a bulkhead is neither in commercial service, nor serving to retain shoreline oil and/or tar contamination, then cannot this wall be dismantled and in its place a tidal wetland constructed? Yes to all – and all these efforts have begun.
The LaGuardia students who first engaged in habitat recovery at their local estuary of Newtown Creek discovered they could suspend buckets of sand into the intertidal zone, plant this sand with cord grass plants acquired from the City Parks Native Plant Center, transfer into the bucket several mussels from the intertidal steps of the NYCDEP Nature Walk at Whale Creek, and witness both plant growth and mussel survival! Single adult mussels, placed onto the sand, used their foot like a clam would use its foot, to burrow next to the small Spartina plants (the grasses were not yet large enough to anchor byssal threads). In sum, habitat suspension works. A “proof of concept” was demonstrated (Fig. 9A).

Figure 9. A: A 2013-14 pilot project of suspended bucket-habitat units (see text) was followed by suspension of steel habitat basins the following year (here at Dutch Kills). B: 2020 winter photo of mussels (starred) in steel basin suspended at North Henry St. inlet. Photos by author.
What next? The City Parks Foundation (CPF), administrator of a NY State Environmental Benefits grant to LaGuardia Community College, appointed a Project Manager, who then contacted Hammersmith Metal fabricators – the firm who did metal work for the High Line in Manhattan. Hammersmith steel basins, of dimension 1.2 m long, 0.5 m wide and 0.75 m deep, were suspended from the walls of Newtown Creek. LaGuardia students, with assistance from volunteers with the Newtown Creek Alliance and North Brooklyn Community Boathouse, lined the new basins with planting cloth (“landscape fabric”), filled the basins with sand and planted each with 60 Spartina alterniflora – or cordgrass -“plugs” (little plants). In the years since, the plants have thrived. And then the mussels came (Fig. 9B).

Figure 10. A: Steel habitat basins supported on I-beam at NYCDEP “Nature Walk” at Whale Creek. B: Former LaGuardia student Nirmela Govinda takes sediment samples for her masters thesis (2021). C: Frame-suspended basins along WM (Waste Management) bulkhead – LIC/Blissville. D: Student Shawn Blackman (CPF fellow) points to design for artificial tidepool using clay pot (2023). Photo A by Willis Elkins, photos B-D by author.
Three sites now host marsh habitat along the walls of the Creek: the NYCDEP bulkhead at the mouth of Whale Creek (Fig. 10 A, B), the Waste Management bulkhead (Fig. 10 C, D) and chain-suspended basins at the North Henry inlet. Former LaGuardia student Nirmela Govinda (Fig. 10B) completed her master’s degree at Brooklyn College by demonstrating the ability of sediment microbes in habitat basins to “denitrify” (remove nitrate, NO3-) from the surrounding water. [8] Nitrate is released into the water by CSO discharges, where it fuels harmful algal blooms.
Thus, it is not only the mussels that can heal the waters of Newtown Creek, but also the microbes in the sediments of the habitat units. Furthermore, as grasses grow within the intertidal basins, they draw the nitrate from the water, incorporating its nitrogen into their vascular tissue and leaving less nitrate to fuel blooms of algal cells.
What of restoring lost habitat also via flotation? Floating subtidal habitat had already been demonstrated at the Harbor School on Governors Island, where a floating dock, the “Eco-dock,” was outfitted with submerged habitat for oysters. Taking this idea, the Newtown Creek Alliance built a “Living Dock,” designed with internal cavities that contained different types of subtidal habitat, e.g., shells, sediment or fabric strips – a stand-in for the eel grass that once grew in the shallow clear waters of the estuary. After the NCA’s “Living Dock” was tethered at the North Henry Street inlet. Willis Elkins, who developed the Dock’s design, suggested planting some of the Spartina grass plugs into sand-filled crates of the Dock. This done, we discovered that Spartina roots can remain continually submerged without affecting the health of the plant.

Figure 11: A. Marsh rafts of cedar wood on bulkhead in 2017 at Newtown Creek Alliance, built with assistance from NCA Exec. Director Willis Elkins (orange cap). Students from left: Marta Candia, Nirmela Govinda, Estrella Casarez. Blue barrels: refurbished food storage containers for flotation. B. Marsh raft internal compartment holds 4 crates. C. Flowering Spartina a. grasses produce wind-pollinated flowers (circled, 2023). Note broad-leaf plants in bordering planters. D. NCA Living Dock at left, marsh rafts at right, in 2024. Black material is landscape fabric. Note insect-pollinated flowers (circled). Photos by author.
Could we design a raft to support both the low marsh Spartina grasses and the insect-pollinated plants of the high marsh, thereby supporting our native pollinators? We could indeed – and to do so, we received a small grant from City Council Member Jimmy Van Bramer to purchase cedar wood (Fig. 11). Cedar is a remarkable wood that resists moisture, rot and insects – but the cedar species most suitable for marine use are also the most at risk from climate change. And cedar is expensive.
In 2020, we began to experiment with designs for inexpensive and biodegradable floating marsh habitat. Most critically, we sought a design offering easy construction that would be suitable for school and community groups to build. For indeed, to have ecosystem impact, pilot projects must be moved from a demonstration phase to system-wide application. The inspiration of student Harry Aguillar, then a CPF Fellow, now a ranger and educator with City Parks, led us to experiment with wood shipping pallets as floating frames. Shipping pallets are abundantly available outside big-box stores and supermarkets; the pallets stamped “HT” for heat-treated are without chemical contamination. Thus far, inquiry and explanation offered at pallet deposit sites have been enough to generate offers of free pallets!
Since 2020, three different pallet-based floating marsh designs have been attempted. The first, based on weaving a bamboo-strip platform beneath the pallet to support glass flotation (thank you to students Jacob Centeno and Harry!) did indeed support a small stand of Spartina grass for two seasons. But in its third year, the installation became top-heavy as its grasses reached 4+ feet and, alas, it capsized. Next up was an attempt to use sheets of cork stuffed between side boards of a pallet. But alas, we discovered that when cork is cut into sheets, it loses buoyancy and becomes water-logged, acting as a weight upon the pallet…. This design didn’t survive a year.

Figure 12. A. Jeremy and Gretchen on NCA Living Dock with pontoons of knotweed stems. B. Pontoons held against pallet with outer wrapping of stainless-steel mesh, inner of coir mat. Onyi inserts Spartina plugs between openings of coir netting. C-E. Mallard duck family discovers pallet. Photos by author.
In 2025, we hit upon a design both stable and maintainable, thanks to students Gretchen Begley, CPF Fellow (also hired by City Parks); Onyi Erondu, a CUNY Research Scholars Program (CRSP) intern; and Jeremy Ceballos, Levin-Goffe Fellow. Dry hollow stems of Japanese knotweed or non-native bamboo (happily for us, invasive “nuisance” plants) can be bundled along opposing sides of a pallet, thereby providing both buoyancy and stability. This design had the additional benefit of being exceedingly attractive to ducks (Fig. 12).
So, a project design may fail or succeed – and if it succeeds, it must be maintained to remain. Since the outset of the effort to return some marsh habitat to the waterway, installations have inevitability been challenged by forces destructive to human constructions; ice and rust, for example (Fig. 13A&B). But these natural processes are our teachers, and we can learn how to respond (as in attaching “sacrificial anodes” to metal installations). More recently, some targeted damage may have befallen basins along the WM bulkhead on the main waterway between the Greenpoint Ave and Kosciusko bridges (Fig. 13, C-G, two examples).

Figure 13. A. Icing of pilot suspension prevented tidal submergence (Dutch Kills). B. Corrosion of basin supports caused collapse (Whale Creek). C. Frame-suspended basin HB-22 rammed by vessel? Apparent trajectory and focal damage suggested not a barge/tug mishap. D. Submerged marsh basin LB-1 supported visiting swimmers (inset:shrimp visitor). E-F. Basin LB-1 found without grasses. Living soft-shelled clams found & returned; clay pot placed as tidepool (stars), basin replanted by student intern Diana Peredes. G. LB-1 again found without grasses. Grass loss coincided with rake (?) marks (arrows). Photo C by Willis Elkins; Photos A,B, E-G by author.
With time, it’s hoped that all members of the waterway’s human communities will embrace the vision of the Newtown Creek Alliance, wherein our waterway supports a beneficial coexistence of both commercial activity and, concurrently, a magnificent community of non-human beings - a community that offers joy of observation and of discovery to workers, residents and visitors to the estuary.
A final note: The beginnings of a constructed wetland are happening at the North Henry Street inlet. The NYS Environmental Benefits Fund, managed by the CPF, awarded over $1M to LaGuardia-CUNY and the NCA to develop a Spartina tidal marsh along the inlet’s shoreline, in collaboration with the environmental services firm Nelson Pope Voorhis and the landscape architecture firm TerrainNYC.
Carter Craft and Michael Mullaley of the City Parks Foundation provided indispensable guidance and materials for the projects here (C.C. now Maritime & Water Advisor, Consulate General of the Netherlands in NY). Thank you! And our thanks to artist, naturalist and former LaGuardia colleague, Priscilla Stadler, who offered her time and skills to developing tidal pool habitat for suspended grass basins.
[1] Biological Productivity: amount of living biomass (measurable as dry weight) produced per unit volume per unit time.
[2] Sanderson, E. W., & The Welikia Project. (n.d.). The Welikia Project: A natural history of New York City. Retrieved October 15, 2025, from https://www.welikia.org.
[3] Eviatar, Daphne. “The Ooze.” New York Magazine, 2007. Retrieved November 9, 2025, from https://nymag.com/news/features/32865/
[4] Burrows, E. G., & Wallace, M. (1999). Gotham: A history of New York City to 1898. Oxford University Press. Chicago: Burrows, Edwin G., and Mike Wallace. Gotham: A History of New York City to 1898. New York: Oxford University Press.
[5] Steinberg, T. (2014). Gotham unbound: The ecological history of greater New York. Simon & Schuster.
[6] Galimany, E., Wilkfors, G. H., Dixon, M. S., Newell, C. R., Meseck, S. L., Henning, D., Li, Y., & Rose, J. M. (2017). Cultivation of the Ribbed Mussel (Geukensia demissa) for nutrient bioextraction in an urban estuary. Environmental Science & Technology, 51, 13311–13318; https://pubs.acs.org/doi/pdf/10.1021/acs.est.7b02838?ref=article_openPDF
[7] Durand, S. E., Niespor, R., Ador, A., Govinda, N., Candia, M., & Torres, K. (2020). Ribbed mussel (Geukensia demissa) in an urban waterway filters bacteria introduced by sewage. Marine Pollution Bulletin, 161, 111629; https://doi.org/10.1016/j.marpolbul.2020.111629
[8] Govinda, N., Groffman, P. M., Durand, S. E., Zarnoch, C. B., & Elkins, W. (2023). Denitrification potential of surface soils of constructed wetlands in Newtown Creek, an urban superfund site. Environmental Science & Technology, 52(4), 837–846; https://doi.org/10.1002/jeq2.20495
Noted invertebrate species of NY/NJ estuary:
| Anemones | |
| Striped Anemone | Diadumene lineata |
| Ghost Anemone | Diadumene leucolena |
| Barnacles | |
| Bay Barnacle | Amphibalanus improvisus |
| Ivory or American Acorn Barnacle | Amphibalanus eburneus |
| Atlantic Marsh Fiddler Crab | Minuca pugnax |
| Ribbed Mussel | Geukensia demissa |
| Soft-shell Clam | Mya arenaria |