by Dr. Shashikanth Ponnala (sponnala@lagcc.cuny.edu)
Over the past two decades, cancer treatment has undergone profound advancements, reshaping the therapeutic landscape. Innovations in molecular biology and immunology have led to the emergence of targeted therapies and immunotherapies, which have transformed conventional cancer management strategies. Targeted therapies inhibit specific molecular pathways or proteins that drive tumor proliferation, survival, and metastasis, while immunotherapies modulate the host immune system to enhance antitumor responses. Despite these breakthroughs, traditional modalities such as surgery, chemotherapy, and radiation therapy remain central components of oncologic care. Among these, radiation therapy continues to play a critical role, with approximately half of all cancer patients receiving it at some stage of their treatment. Traditional radiation therapy involves external beam techniques to deliver ionizing radiation from outside the body to destroy tumors within, however, recent advances in radiation field enabled more precise, image-guided treatment [1a, 1b].
Radiation therapy is a cornerstone of modern cancer treatment, used in approximately half of all cancer patients. With advancements in precision imaging and targeted radiation delivery, Radio Ligand Therapy [2a] (RLT) has emerged as a next-generation [2b] precision medicine strategy that integrates the specificity of molecular targeting with the therapeutic potential of radiation. RLT involves the selective delivery of radioactive dose to tumor cells using molecules designed to bind specific extra cellular targets (receptors). In some cases, certain radionuclides possess intrinsic affinity for tissues or metabolic pathways, allowing for targeted accumulation without extensive chemical modification. One of the primary advantages of RLT lies in its ability to selectively irradiate cancer cells including those in metastatic or inaccessible regions while minimizing damage to surrounding healthy tissue. Furthermore, RLT can be administered systemically, locally, or physiologically, enabling internal radiation delivery rather than external application.
Radio ligands also can be used as Imaging agent [3] for diagnostic purposes by just switching the short-lived radionuclides (68Ga t1/2=68 min, 64Cu t1/2=12.7 h) prior to therapy to visualize and quantify radiopharmaceutical uptake in tumors vs other organs, allowing clinicians to predict therapeutic efficacy and personalize treatment dosing. Compared with conventional cancer therapies, RLT offers high efficacy, improved safety, and reduced toxicity profiles [3b, 3c]. The recent approval of multiple radiopharmaceutical agents (Lutathera, Pluvicto, Zevalin) by the U.S. Food and Drug Administration underscores the growing clinical relevance and therapeutic promise of this modality. This short review aims to provide a quick overview and applications of Radio Ligand Therapy in cancer treatment, including its mechanism.
Radioligand therapy utilizes a bifunctional ligand [4a] comprised of a pharmacophore (often a small molecule, peptide or antibody) that selectively binds a tumor-associated target, and a chelator that traps the radio nuclide emitting particle radiation (β-, α-) when decay. Upon binding and internalization (or retention) in tumor cells, the radionuclide delivers lethal radiation within the microenvironment of cancer cells. The “theranostic” concept first performing a diagnostic imaging step (e.g., PET or SPECT) using the same or similar ligand labelled with a diagnostic radionuclide, followed by therapeutic administration allows for patient selection, dosimetry planning and treatment monitoring. For example, in the case of prostate cancer [4], a ligand targeting the Prostate Specific Membrane Antigen (PSMA) may first be labelled with a positron (β+) emitter for imaging like 68Ga or 64Cu and then with a β-emitter (177Lu) or α-emitter (225Ac) for therapy. RLT thereby bridges molecular imaging and molecular therapy.
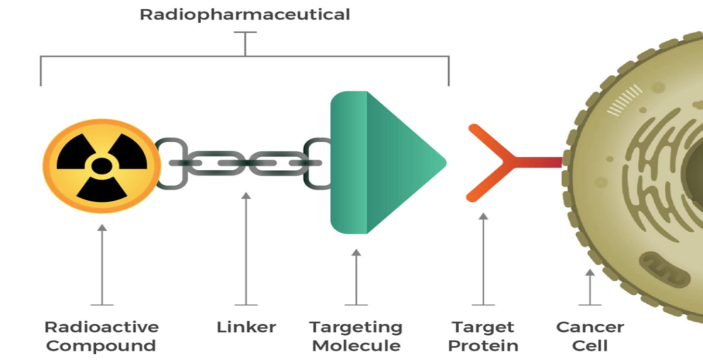
Figure 1: Radioligands consist of a radioactive payload and a targeting molecule joined by a linker that binds to cancer-cell targets [4b].
One of the foremost clinical successes of radioligand therapy (RLT) has been observed in the treatment of metastatic castration-resistant prostate cancer (mCRPC). Among the Radio ligands developed, Lutetium-177 – labeled PSMA-617 (177Lu-PSMA-617, brand name: Pluvicto®, Fig. 2) has emerged as a landmark therapeutic option [5a, 5b]. This agent specifically targets the prostate-specific membrane antigen (PSMA), a transmembrane protein overexpressed on prostate cancer cells, enabling selective delivery of beta-emitting radiation to malignant tissue while minimizing damage to surrounding healthy structures. Clinical trials have demonstrated that 177Lu-PSMA-617 significantly improves both progression-free survival and overall survival in patients with PSMA-positive mCRPC, including those who have progressed following conventional androgen receptor pathway inhibitors and taxane-based chemotherapy [6a, 6b]. Without a doubt, 177Lu-PSMA therapy offers meaningful clinical benefit in terms of tumor response, biochemical reduction in prostate-specific antigen (PSA) levels, which led to significant improvement in both overall and progression-free survival.
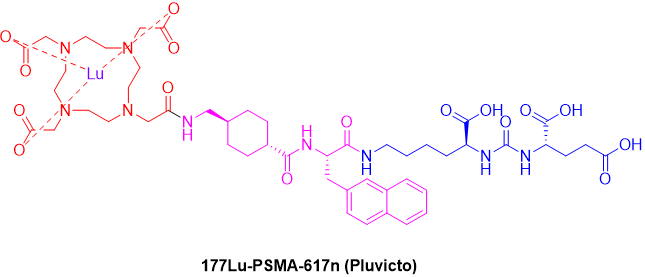
Figure 2: Chemical Structure of Pluvicto.
Another well-established and clinically validated indication for RLT is in the treatment of well-differentiated neuroendocrine tumors [7a] (NETs). These tumors are characterized by the overexpression of somatostatin receptors (SSTRs), which enables selective targeting by radiolabeled somatostatin analogues. The agent 177Lutetium–DOTATATE (Lutathera®, Fig. 3) represents a cornerstone in this therapeutic class, combining the high-affinity somatostatin analogue octreotate with the beta-emitting radionuclide lutetium-177 to deliver cytotoxic radiation directly to receptor‐expressing tumor cells while sparing normal tissues.
The clinical efficacy and safety of 177Lu-DOTATATE were explored in demonstrated in the clinical trial of patients [7b] with progressive and metastatic NETs. Participants received 177Lu-DOTATATE showed a remarkable improvement in progression-free survival (PFS). Beyond the NETTER-1 (NeuTech Endocrine Radionuclide Therapy Evaluation Randomized, 1) clinical trial, multiple academic and independent hospital research studies have confirmed the reproducibility of these benefits across broader NET populations.
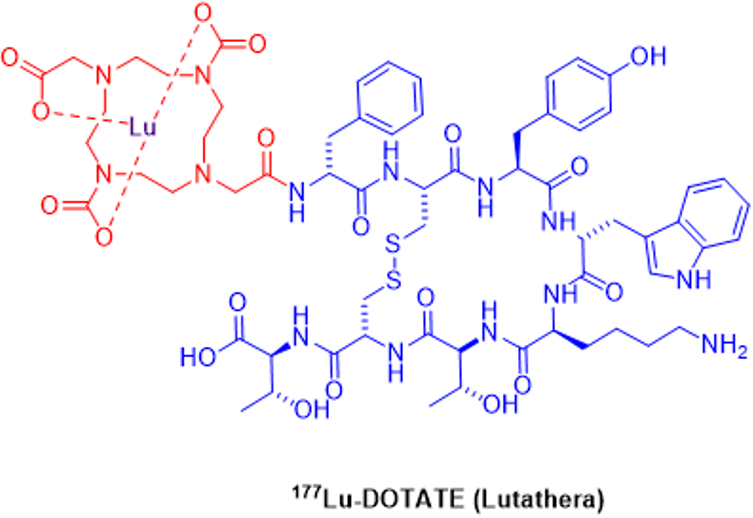
Figure 3: Chemical Structure of Lutathera.
All these evidence supports 177Lu-DOTATATE as a highly effective, and well-tolerated therapy that has transformed the therapeutic landscape for GEP-NETs. It stands as a classic example of theragnostic in oncology, leveraging molecular imaging with 68Ga-DOTATATE PET/CT for [7c] precise patient selection and therapy monitoring, thereby embodying the shift toward precision-guided radionuclide therapy.
Despite of various well established uses, RLT is currently being investigated across a broad spectrum of tumor types and molecular targets, reflecting the rapid expansion of this therapeutic modality. Ongoing research is exploring its potential in malignancies that extend well beyond traditional neuroendocrine and prostate cancer indications. A recent comprehensive review [2a] identified over 400 registered clinical trials worldwide (Fig. 4) assessing novel targets for RLT. These include the Fibroblast Activation Protein (FAP), which is abundantly expressed in cancer-associated fibroblasts within the tumor stroma; carbonic anhydrase IX (CAIX), a hypoxia-related enzyme overexpressed in several solid tumors such as renal cell carcinoma; and the gastrin-releasing peptide receptor (GRPR), which is implicated in the growth and signaling pathways of various epithelial cancers, including breast, lung, and prostate malignancies. Collectively, these investigations highlight the growing interest in expanding RLT to a wider array of tumor types and in refining its application through molecularly targeted approaches [2a].
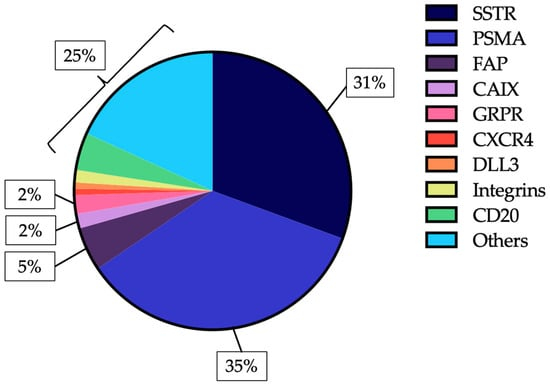
Figure 4: RLT molecular targets in clinical trials [2a].
Radioligand therapy (RLT) has several key benefits [8] that make it an important and promising cancer treatment. It uses ligand–radionuclide compounds to deliver radiation directly to tumor cells and metastases, including those that cannot be reached by surgery or external radiation. Because it works systemically, RLT can target both visible and microscopic disease inside the body. Its theranostic approach using the same molecule for imaging and treatment allows for personalized planning and monitoring of therapy. By focusing radiation within tumors, RLT also helps protect healthy tissues, reducing side effects compared to standard treatments. Recent studies suggest it may be effective when used earlier in the disease course; for example, in aggressive neuroendocrine tumors, early RLT extended progression-free survival from about 8.5 to 22.8 months [9].
Radioligand therapy (RLT) is expected to grow and improve in several ways. New radionuclides and ligands, such as α-emitters [10] and molecules designed for better tumor targeting or faster clearance from healthy tissue, aim to make treatments more effective and precise. Advances in personalized dosing using patient-specific imaging and monitoring could allow truly tailored therapy. Combining RLT with immunotherapy [11], chemotherapy, or targeted drugs also shows promise for better outcomes and overcoming treatment resistance.
In conclusion, radioligand therapy (RLT) is a breakthrough in cancer care, combining both diagnosis and treatment in a precise, personalized way. With the approvals for prostate cancer and neuroendocrine tumors and many new treatments in development, RLT is moving from a last-resort option to a more widely used therapy. To reach its full potential, improvements are needed in patient selection, dosing, combination with other treatments, healthcare infrastructure, and access worldwide. As these challenges are met, RLT is likely to become a key part of cancer treatment, offering more effective, tailored, and safer therapies for patients.
[1a] D. E. Gerber and J. D. Minna, “Targeted therapies and their impact on cancer treatment,” J Clin Oncol, 32(11): 1452–1460, (2014).
[1b] C. Allen, S. Her, and D. A. Jaffray, “Radiotherapy for Cancer: Present and Future,” Adv Drug Deliv Rev, 109: 1–2, (2017).
[2a] G. Ninatti, S. T. Lee, and A. Scott, “Radioligand Therapy in Cancer Management: A Global Perspective,” Cancers, 17(21): 3412, (2025).
[2b] B. J. B. Nelson, V. Krol, A. Bansal, J. D. Andersson, F. Wuest, and M. K. Pandey, “Aspects and prospects of preclinical theranostic radiopharmaceutical development,” Theranostics, 14(17): 6446–6470, (2024).
[3a] L. Solnes, R. A. Werner, K. M. Jones, M. S. Sadaghiani, C. R. Bailey, C. Lapa, M. G. Pomper, and S. P. Rowe, “Theranostics: Leveraging Molecular Imaging and Therapy to Impact Patient Management and Secure the Future of Nuclear Medicine,” J Nucl Med, 61(3): 311–318, (2020).
[3b] Calais J, Czernin J, Thin P, Gartmann J, Nguyen K, Armstrong WR, Allen-Auerbach M, Quon A, Bahri S, Gupta P, Gardner L, Dahlbom M, He B, Esfandiari R, Ranganathan D, Herrmann K, Eiber M, Fendler WP, Delpassand E., “Safety of PSMA-Targeted Molecular Radioligand Therapy with 177Lu-PSMA-617: Results from the Prospective Multicenter Phase 2 Trial RESIST-PC (NCT03042312),” J Nucl Med. 62(10):1447-1456, (Oct. 2021).
[3c] Keerti Sitani, Rahul Parghane, Sanjay Talole, Sandip Basu, “The efficacy, toxicity and survival of salvage retreatment PRRT with 177Lu-DOTATATE in patients with progressive NET following initial course of PRRT,” British Journal of Radiology, Volume 95, Issue 1137, 1, 20210896 (Sept 2022).
[4a] H. Duan, A. Iagaru, and C. Aparici, “Radiotheranostics – Precision Medicine in Nuclear Medicine and Molecular Imaging,” Nanotheranostics, 6(1): 103–117, (2022).
[5a] M. S. Hofman, L. Emmett, et al., “[177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomized, open-label, phase 2 trial,” Lancet Oncol, 22(9): 1173–1184, (2021).
[5b] F. Edler von Eyben, I. Virgolini, and R. Baum, “Review on the Increasing Role for PSMA-Based Radioligand Therapy in Prostate Cancer,” Cancers, 16(14): 2520, (2024).
[6a] S. Satapathy, R. K. Sahoo, and C. Bal, “177Lu-PSMA Radioligand Therapy Efficacy Outcomes in Taxane-Naïve vs Taxane-Treated Patients with mCRPC: Systematic Review & Meta-analysis,” J Nucl Med, 64(8): 1266–1271, (2023).
[6b] S. Swayamjeet, K. S. Ranjit, and B. Chandrasekhar, “[177Lu]Lu-PSMA-Radioligand Therapy Efficacy Outcomes in Taxane-Naïve Versus Taxane-Treated Patients with Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-analysis,” J Nucl Med, 64(8): 1266–1271, (2023).
[7a] J. Strosberg, G. El Haddad, E. Wolin, A. Hendifar, J. Yao, B. Chasen, et al., “Phase 3 trial of 177Lu-DOTATATE for midgut neuroendocrine tumours,” N Engl J Med, 376(2): 125–135, (2017).
[7b] E. S. Delpassand, et al., “177Lu-DOTATATE in Patients with Progressive Neuroendocrine Tumors: A Retrospective Real-World Study in the United States,” J Nucl Med, 65(5): 746–752, (2024).
[7c] R. A. Werner, C. Bluemel, M. S. Allen-Auerbach, et al., “68Gallium- and 90Yttrium/177Lutetium: ‘theranostic twins’ for diagnosis and treatment of NETs,” Ann Nucl Med, 29: 1–7, (2015).
[8] L. Urso, A. Nieri, I. Rambaldi, et al., “Radioligand therapy (RLT) as neoadjuvant treatment for inoperable pancreatic neuroendocrine tumors: a literature review,” Endocrine, 78: 255–261, (2022).
[9] Novartis Press Release, “Lutathera® significantly reduced risk of disease progression or death by 72% as first-line treatment in advanced GEP-NETs,” (2023). Available from: Novartis website.
[10] E. Ostuni and M. R. G. Taylor, “Commercial and business aspects of alpha radioligand therapeutics,” Front Med (Lausanne), 9: 1070497, (2023).
[11] R. Jiao and E. Dadachova, “Combination of Radioligand Therapy and Immunotherapy: How to Make It Work in Clinic?” Immunotargets Ther, 14: 755–759, (2025).