by Afsana Abdul Rahim*, Sarah Seron*, Jacob Martinez* and Dr. Amit Aggarwal
This research project was done by LaGuardia students Afsana Abdul Rahim, Sarah Seron, and Jacob Martinez as a part of the Honors "General chemistry II" class. *A. A. R., S. S. and J. M. contributed equally to this work. The project investigates porphyrin supramolecular systems.
Instructor: Dr. Amit Aggarwal, Natural Sciences department, LaGuardia/CUNY
link to the file: porphyrin.pdf (382K)
Abstract
Porphyrin supramolecular systems are well proven to serve as promising components for advanced materials because of their unique physicochemical, optoelectronic, and magnetic properties. Here we present the colloidal nanoparticle solution of a free base 5,10,15,20-tetrakis(pentafluorophenyl) porphyrin, TPPF20, using mixed solvent method. The formation of nanoparticles was confirmed by the opaqueness of solution and also by UV-visible absorption spectroscopy. The broadening of Soret band in the range of 370-480 nm as well as the redder shift in low energy Q-bands is an indicative of aggregation of porphyrin to form their nanoparticles solution. Light scattering experiment, Tyndall Effect, also proves the formation of bigger particles in the colloidal solution.
The studies on synthesis and application of organic nanoparticles is relative new compare to the inorganic nano-materials composed of metals, metal oxides, ceramics etc. [3, 4] and porphyrinoids are at the forefront of this research. [5-7] The methods used for the preparation of both inorganic as well as organic nanomaterials involves strong experimental conditions, toxic chemicals and special instrumentation/techniques that all limits the development of advanced materials for their applications for modern society.[4] Therefore, there is a strong need to develop a simple and cost-effective method to prepare nanomaterial for their applications.
Here, we are presenting a simple, quick and cost-effective method to prepare organic nanoparticles of a free base 5,10,15,20-tetrakis(pentafluorophenyl) porphyrin, TPPF20, by mixing host-guest solvent method reported earlier by Drain and coworkers [5, 6]. The structure of 20 is shown in figure 1b. The formation of colloidal nanoparticles was confirmed by opaqueness of the solution, absorption spectra and also by scattering of light, that indicates the presence of small particles in solution.
Materials: 5,10,15,20-tetrakis(pentafluorophenyl) porphyrin (TPPF20),Tetrahydrofuran (THF) and polyethyleneglycol monomethylether (PEG164) were analytical grade and purchased from Sigma Aldrich Chemicals Co.
Preparation of Colloidal solution of organic nanoparticles (ONPs): A stock solution of TPPF20 was prepared by dissolving 2 mg of it in 2 ml THF (1mM solution). Then 400 μL portion of this stock solution of TPPF20 in THF was mixed with 200 μL of polyethylene glycol, PEG164 in a 10 mL vial at room temperature. To this mixture solution then 5.0 mL of distilled water was added while magnetic stirring over a time period of 60 seconds and then the solution was further stirred under the same environmental conditions for another 2-3 minutes.[6, 8]
UV-visible Absorption Spectroscopy: The absorption spectra were recorded on Cary Bio-3 UV/Vis spectrophotometer. UV-visible absorption spectra of the stock solution of TPPF20 in THF and the corresponding nanoparticles in water-THF mixed solvent system were recorded using baseline with THF and water respectively.
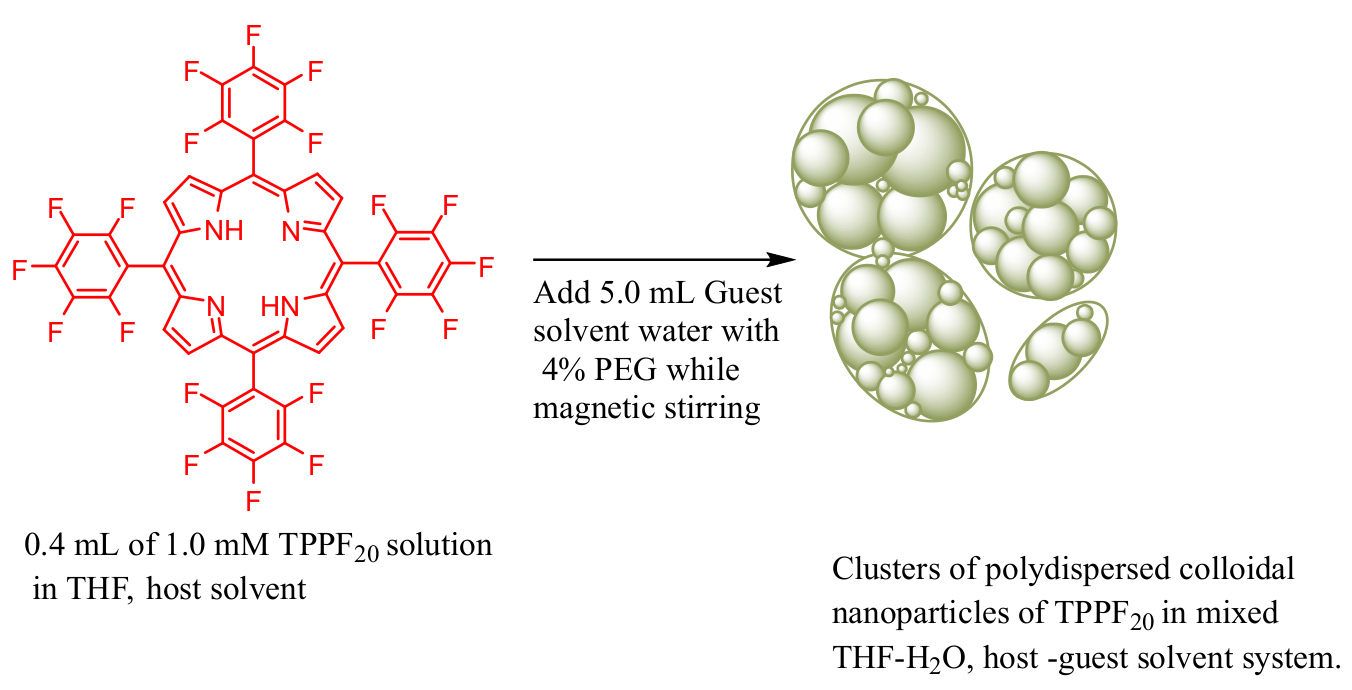
Preparation of ONPs of TPPF20: Mixing of miscible host-guest solvent method is used to prepare a colloidal nanoparticle solution of TPPF20, a free base porphyrin at room temperature. To 0.4 mL portion of a stock solution (1 mM) of TPPF20 in THF, a small amount (0.2 mL) of PEG as stabilizer was added. PEG serves as a bridge between the organic solution of TPPF20 in THF and water and causes the stabilization of the nanoparticles formed. A schematic representation that shows the formation of clusters of colloidal nanoparticles of TPPF20 in miscible host THF and guest water solvent system is shown in scheme 1.

Characterization for colloidal organic nanoparticle formation:
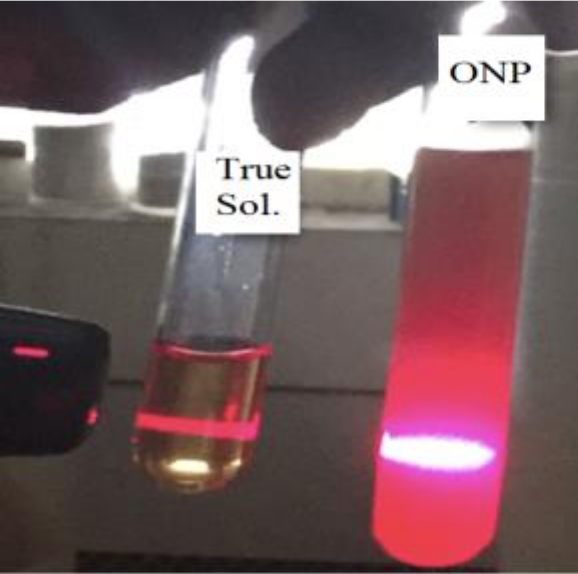
(a) Visual confirmation: A true solution of TPPF20 in THF is clear and transparent while its colloidal solution in miscible solvent system is opaque. The opaqueness of the colloidal solution is indicative of the aggregation of small particles in the colloidal solution (figure 2).
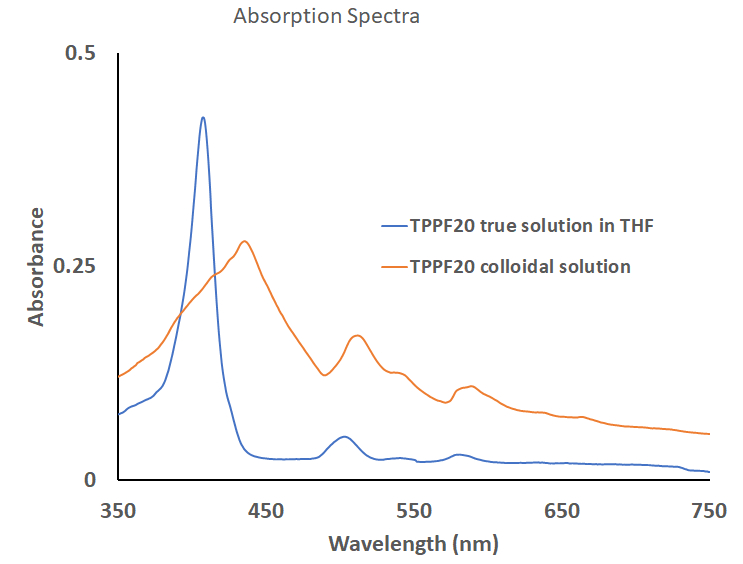
(c) UV-visible absorption spectra: Absorption spectra of both colloidal nanoparticles solution and true solution of TPPF20 in THF were recorded and compared, Figure 4. A strong sharp absorption peak at 406 nm (Soret band) observed for component TPPF20 molecule in THF gets broadened between 370-470 nm range shows the aggregation of component porphyrin molecules to make their nano clusters. Also, the bathochromic shift for both the Soret band peak as well as the four low energy Q-bands for colloidal nanoparticles is indicative of agglomeration of the porphyrins into its colloidal solution. Appearance of small shoulders on both side of the broadened soret band.
In conclusion, we have used a previously reported [6, 8] miscible host-solvent solvent method for the preparation of colloidal nanoparticles of TPPF20 a free base porphyrin under ambient conditions. The significance of this method lies in its simplicity and low-cost instrumentation that makes this method to be ideally to be used by both academia and industrially for other component molecules. Formation of opaque colloidal solution and light scattering is an indicative of formation of porphyrin nanoparticles. The stability of the nanoparticles depends upon the presence of stabilizer. In the absence of stabilizer porphyrin particles aggregates quickly in THF-water solvent system to form its precipitate.
[1] C. Drain, J. Batteas, G. Flynn, T. Milic, N. Chi, D. Yablon, H. Sommers, Designing
Supramolecular Porphyrin Arrays That Selforganize into Nanoscale Optical and Magnetic
Materials, Proc. Nat. Acad. Sci., 99 (2002) 6498-6502.
[2] C.M. Drain, I. Goldberg, I. Sylvain, A. Falber, Synthesis and Applications of
Supramolecular Porphyrinic Materials, in: A.D. Schlüter (Ed.) Functional Molecular
Nanostructures: -/-, Springer Berlin Heidelberg, Berlin, Heidelberg, 2005, pp. 55-88.
[3] A. Didier, L. Feng, A.J. Ruiz, Nanoparticles as Recyclable Catalysts: The Frontier
between Homogeneous and Heterogeneous Catalysis, Angew. Chem. Int. Ed., 44 (2005) 7852-7872.
[4] R. Ladj, A. Bitar, M. Eissa, Y. Mugnier, R. Le Dantec, H. Fessi, A. Elaissari,
Individual inorganic nanoparticles: Preparation, functionalization and in vitro biomedical
diagnostic applications, J. Mater. Chem. B, , 1 (2013) 1381-1396.
[5] C.M. Drain, G. Smeureanu, S. Patel, X. Gong, J. Garno, J. Arijeloye, Porphyrin
nanoparticles as supramolecular systems, New J. Chem., 30 (2006) 1834-1843.
[6] X. Gong, T. Milic, C. Xu, J.D. Batteas, C.M. Drain, Preparation and Characterization
of Porphyrin Nanoparticles, J. Am. Chem. Soc., 124 (2002) 14290-14291.
[7] H. Dieter, R. Jens, Organic Nanoparticles in the Aqueous Phase-Theory, Experiment,
and Use, Angew. Chem. Int. Ed., 40 (2001) 4330-4361.
[8] G. Smeureanu, A. Aggarwal, C.E. Soll, J. Arijeloye, E. Malave, C.M. Drain,
Enhanced Catalytic Activity and Unexpected Products from the Oxidation of Cyclohexene by
Organic Nanoparticles of 5,10,15,20-Tetrakis-(2,3,4,5,6-pentafluorophenyl)porphyrinatoiron(III)
in Water by Using O2, Chem. Eur. J., 15 (2009) 12133-12140.
[9] A. Eisfeld, J.S. Briggs, The J- and H-bands of organic dye aggregates, Chem. Phys.,
324 (2006) 376-384.